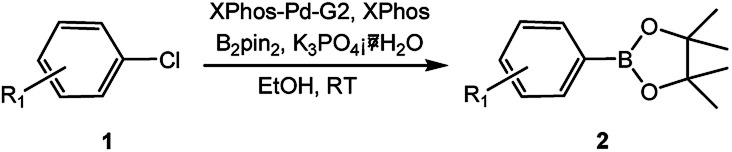
Palladium-catalyzed borylation of aryl chlorides at room temperaturea.
| ||||
|---|---|---|---|---|
| Entry | Ary chloride | Pd (mol%) | Time (h) | Yieldb (%) |
| 1 |

|
0.5 | 0.5 | 92 |
| 2 |
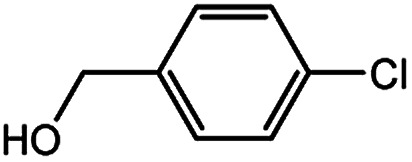
|
0.5 | 2.0 | 96 |
| 3 |

|
0.5 | 1.0 | 74 |
| 4 |

|
0.5 | 1.0 | 84 |
| 5 |

|
0.5 | 1.0 | 72 |
| 6 |

|
0.5 | 4.0 | 83c |
| 7 |

|
1.0 | 8.0 | 85c |
| 8 |

|
1.0 | 6.0 | 82c |
| 9 |

|
0.5 | 1.0 | 93 |
| 10 |
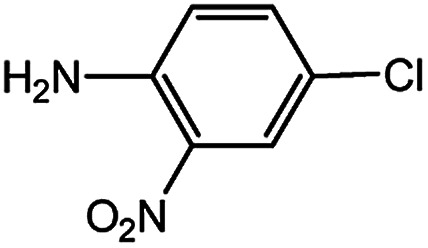
|
1.0 | 1.5 | 63 |
| 11 |

|
1.0 | 3.0 | 67 |
| 12 |
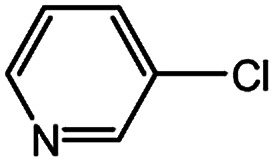
|
0.5 | 0.5 | 90 |
| 13 |

|
0.5 | 0.5 | 88 |
Reaction conditions: 1 (1.0 equiv.), B2pin2 (1.2 equiv.), K3PO4·7H2O (3.0 equiv.), XPhos-Pd-G2 (0.5 mol%), XPhos (0.25 mol%), EtOH (0.5 M).
Isolated yield.
XPhos-Pd-G2 (1.0 mol%), XPhos (0.5 mol%).
