Comparison of forward-order and reverse-order strategies in synthesis of unsymmetrical biaryl compoundsa.

| |||||
|---|---|---|---|---|---|
| Entry | Ar1–Cl | Ar2–Cl | Product | Forward yieldb (%) | Reverse yieldb (%) |
| 1 |

|

|

|
87 | 98 |
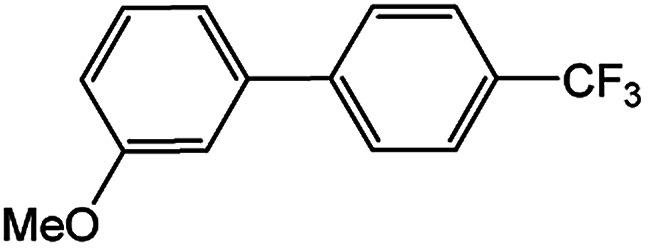
| 2 |

|

|
|
84 | 25 |
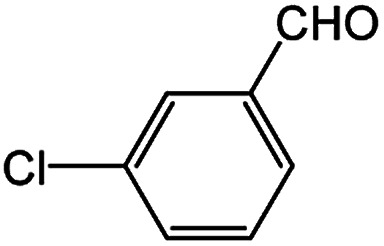
| 3 |

|
|

|
65 | 93 |
| 4 |

|

|

|
91c | 20 |
| 5 |

|

|

|
77c | 42c |
| 6 |

|

|

|
38d | 79c |
| 7 |

|

|

|
35c | 85c |
General conditions: (a) first chloride (1.1 equiv.), XPhos-Pd-G2 (1.0 mol%), XPhos (1.0 mol%), B2pin2 (1.2 equiv.), K3PO4·7H2O (3.0 equiv.), EtOH (0.275 M), RT, 2 h; (b) second chloride (1.0 equiv.), 3.0 M aq. K3PO4·7H2O (3.0 equiv.), RT, 8 h.
Yield of isolated product.
15 h for the second step.
6 h for the second step.
