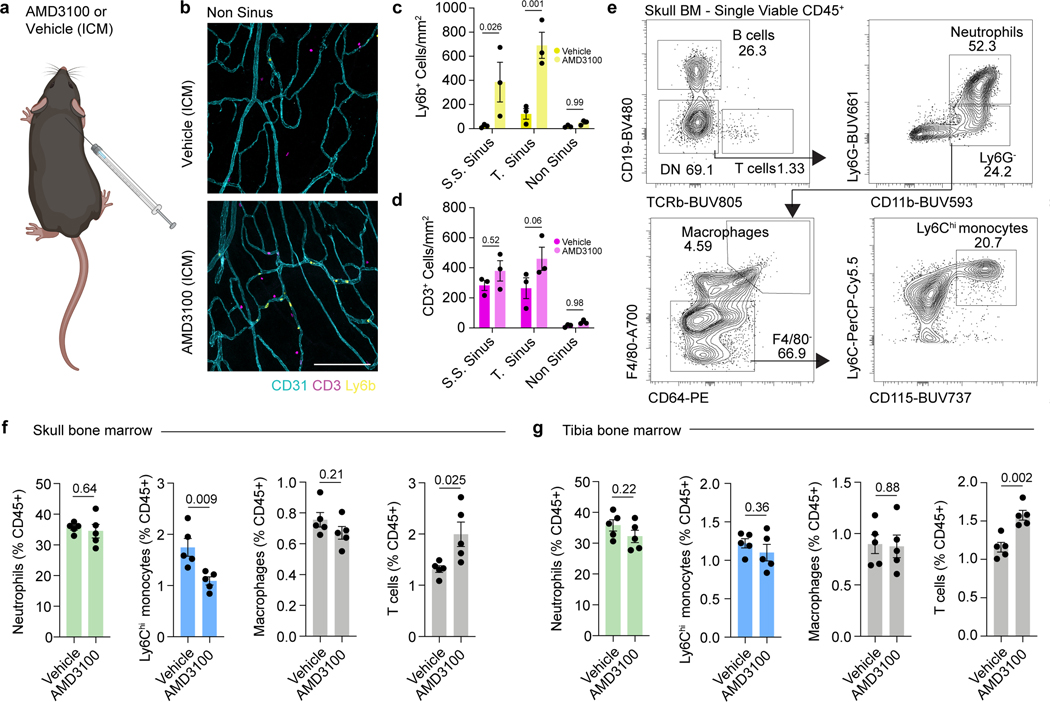
Extended Data Fig. 3. Effects of AMD3100 on immune cell composition of the dura and bone marrow.
a, Experimental design for injections for skull bone marrow egress experiments. AMD3100 (10 μg) or artificial cerebrospinal fluid (aCSF) was injected intra-cisterna magna (i.c.m.), and mice were left for 24 hours. The following day, tissues were processed for immunolabeling or flow cytometry. b, Representative images of Ly6b+ cells and CD3+ cells in non-sinus regions of the dura. Scale bar: 200 μm. c, d, Regional analysis of Ly6b+ myeloid and CD3+ cells in the dura following AMD3100 administration. n = 3 mice per group. Data are means ± SEM, p values represent two-way ANOVA with Sidak’s post hoc test. e, Flow cytometry gating strategy for neutrophils, Ly6Chi monocytes, macrophages, and T cells in the bone marrow following AMD3100 administration. f, g, Relative numbers of neutrophils, Ly6Chi monocytes, macrophages, and T cells in the skull and tibial bone marrow 24 hours following i.c.m. AMD3100 administration. n = 5 mice per group. Data are means ± SEM, p values represent a two-sided Student’s t test.