Fig. 2 |. Targeting astrocyte signalling in Alzheimer disease.
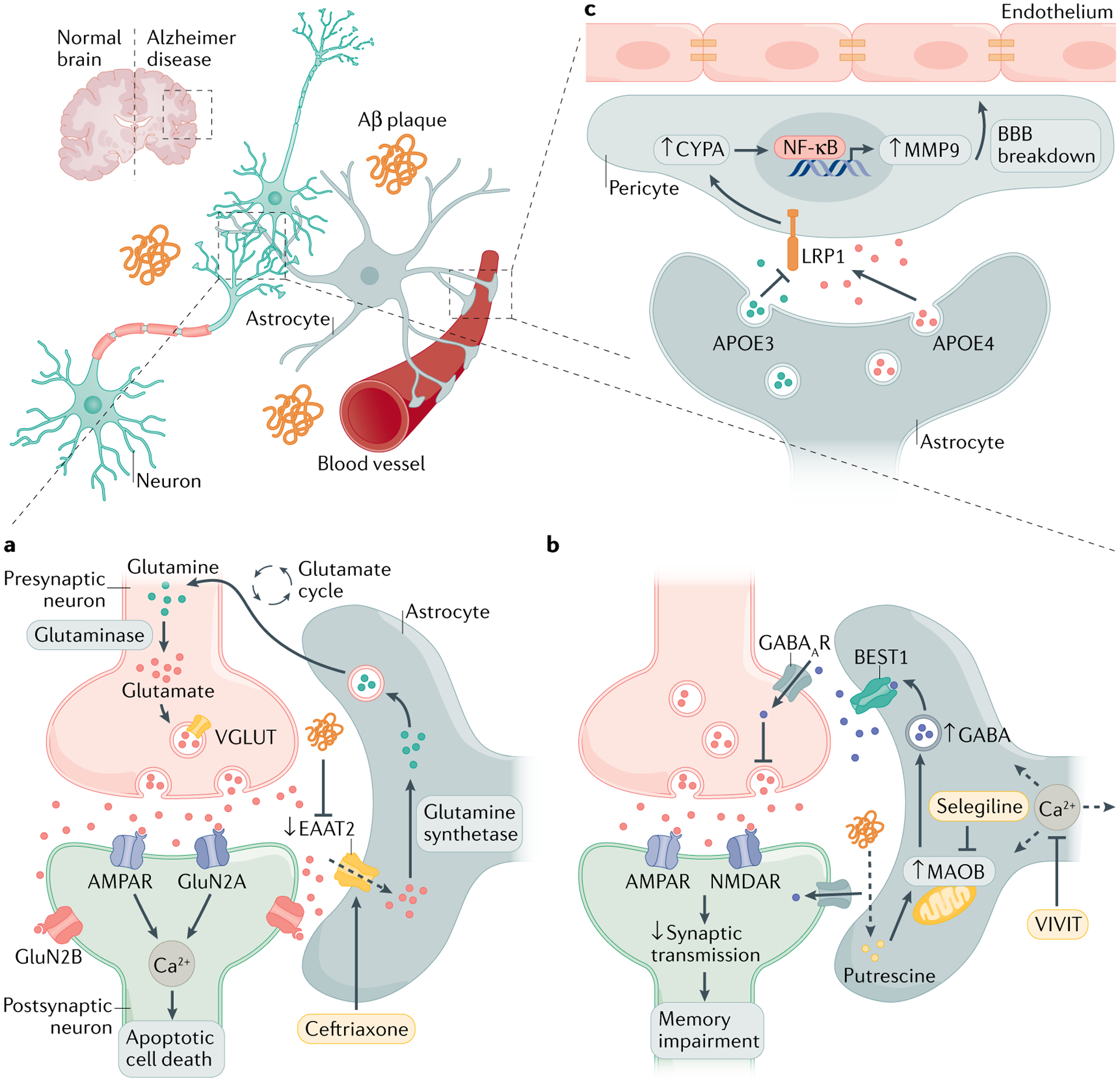
a | The accumulation of amyloid-β (Aβ) in the brain modulates glutamate uptake in astrocytes by inhibiting the glutamate transporter excitatory amino acid transporter 2 (EAAT2). This causes excessive activation of neuronally expressed glutamate receptors, which increases intracellular Ca2+ and promotes neuronal dysfunction. b | Enhanced intracellular Ca2+ waves in astrocytes increase the release of γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain. Aβ plaques and putrescine trigger monoamine oxidase B (MAOB)-mediated GABA release via the Ca2+-activated anion channel bestrophin 1 (BEST1). Increased GABA uptake by neurons impairs memory and synaptic plasticity. c | Astrocytes are the main producers of apolipoprotein E (APOE), which modulates blood–brain barrier (BBB) permeability. APOE4 secretion induces cyclophilin A (CYPA)–nuclear factor-κB (NF-κB)–metalloproteinase 9 (MMP9) signalling in pericytes, which leads to BBB breakdown via the lipoprotein receptor-related protein 1 (LRP1). This APOE4-mediated pericyte activation can be negatively regulated by the production of APOE3. Strategies to rescue neuronal dysfunction mediated by glutamate dysregulation and synaptic plasticity include controlling astrocyte glutamate uptake, Ca2+ homeostasis and GABA production. AMPAR, AMPA receptor; NMDAR, N-methyl-d-aspartate receptor; VGLUT, vesicular glutamate transporter.
