Figure 2, related to Figure S1. Stability and thermal aggregation of γS wild-type and each variant.
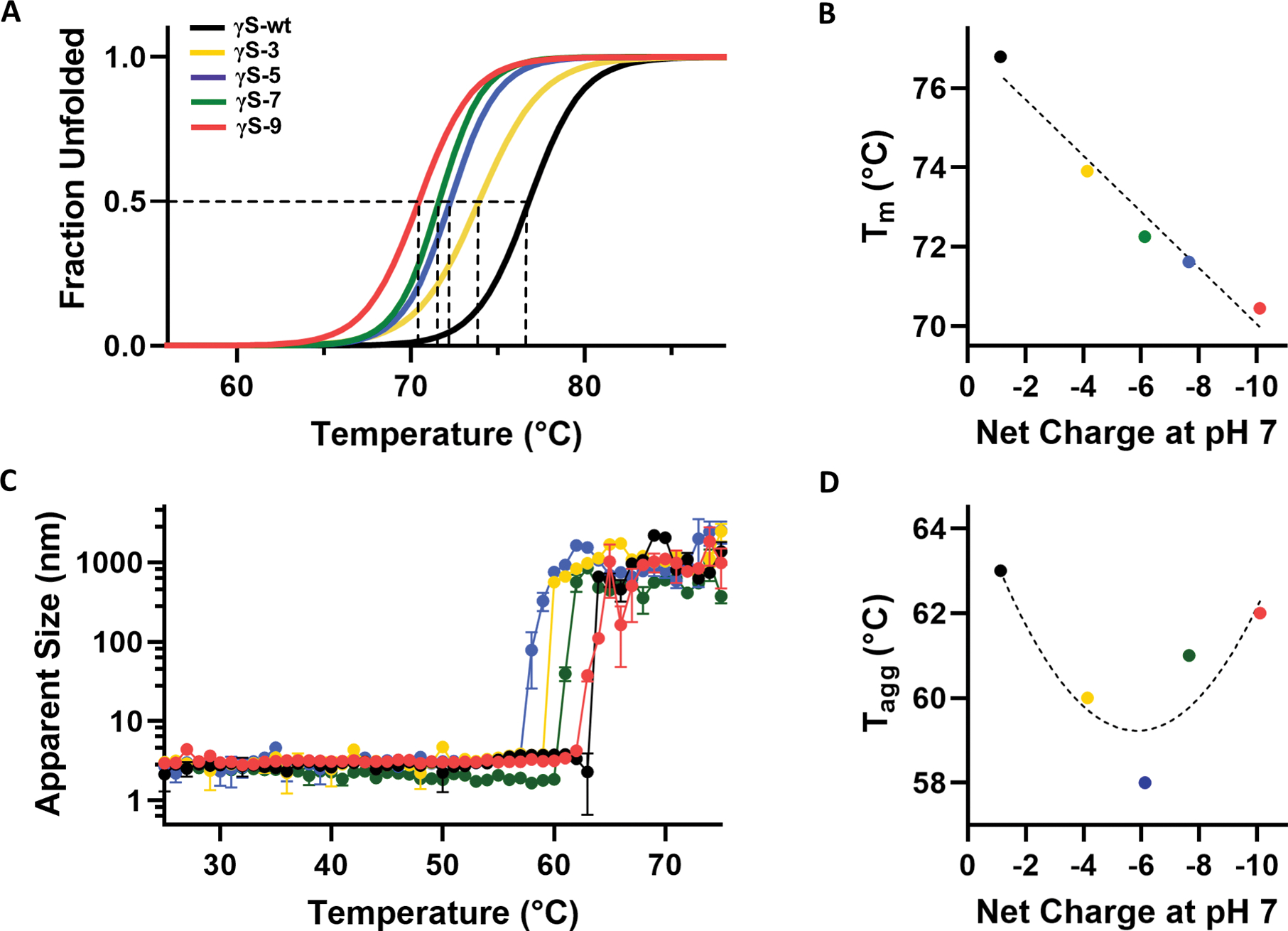
(A) Differential scanning fluorimetry (DSF) was used to determine the percent unfolded as a function of temperature for γS-wt (black), γS-3 (yellow), γS-5 (blue), γS-7 (green), and γS-9 (pink). (B) The midpoint temperature of the thermal unfolding (Tm) of γS-wt and each variant plotted against the net charge of the protein at neutral pH. (C) Dynamic light scattering (DLS) measurements of γS-wt and each variant to monitor thermally induced aggregation of γS wild-type and each variant. Measurements were performed in triplicate. Data are represented as mean ± SD. (D) The temperature of aggregation onset (Tagg) plotted against the net charge of the protein at neutral pH.
