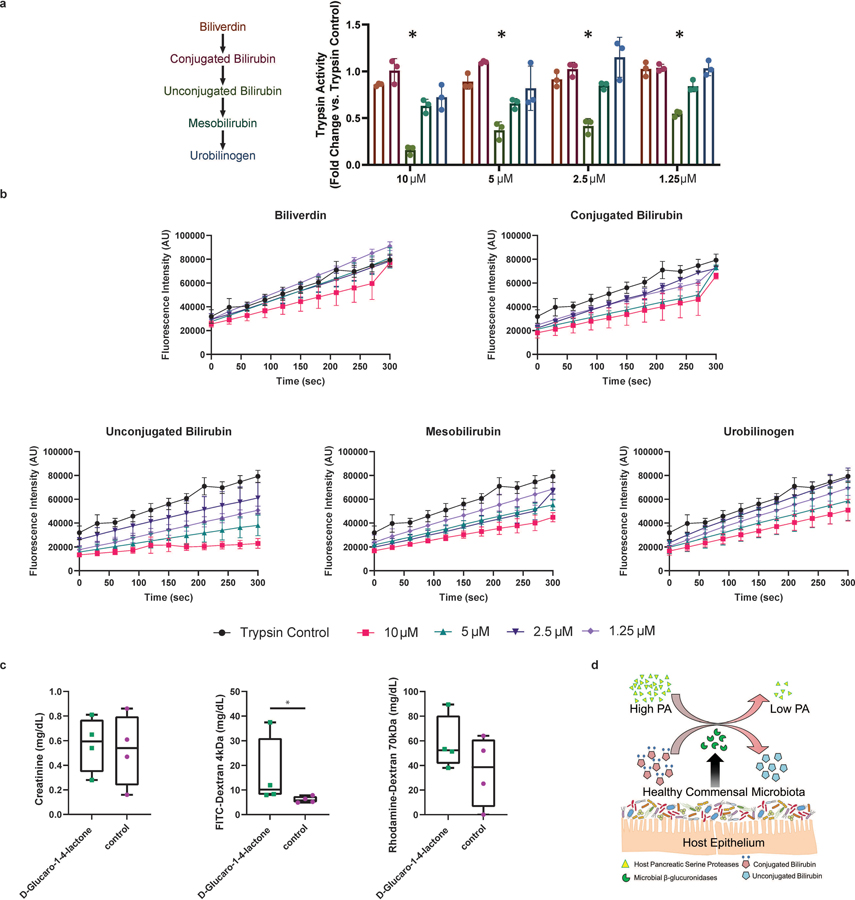
Extended Data Fig. 7. In vitro trypsin activity is suppressed by unconjugated bilirubin, and inhibition of GUS enzymes results in increased intestinal permeability.
a, Trypsin activity in the presence of metabolites within the bilirubin deconjugation pathway. Compared to the other metabolites used for experimentation, unconjugated bilirubin was the only metabolite that significantly inhibited trypsin activity across all concentrations examined. Data presented as Δfluorescence/time, normalized to a trypsin-only control (2-Way ANOVA, Tukey’s multiple comparison test, n=3 *p=0.001, data presented as mean ±SD). b, Time course inhibition of trypsin activity in the presence of bilirubin metabolites (n=3 biologically independent replicates, data presented as mean ±SD). c, Measurement of intestinal permeability in D-Glucaro-1,4-lactone treated humanized mice. Serum 4-kDa FITC-dextran levels were greater in healthy humanized mice treated with D-Glucaro-1,4-lactone indicating inhibition of GUS enzymes causes an increase in leak pathway permeability (2-sided Mann-Whitney, n=4/group *p=0.03). Boxplots: lower, middle and upper hinges correspond to 25th, 50th and 75th percentiles. Upper and lower whiskers extend to the largest and smallest value no further than 1.5 * IQR from the respective hinge. d, Proposed mechanism of microbial based inhibition of host proteases via the production of GUS enzymes.