The scope of amide-forming reactions of carboxylic acids 5 with amines 6.
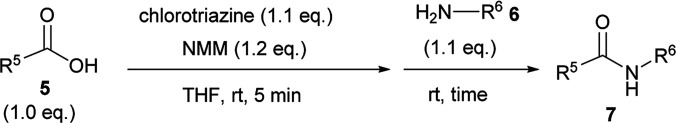
| ||||||
|---|---|---|---|---|---|---|
| Entry | Chlorotriazine | Carboxylic acid 5 | Amine 6 | Amide 7 | Time | Yielda (%) |
| 1 | 2A | 5a | 6a | 7a | 40 min | 91 (83) |
| 2 | CDMT | 40 min | 86 | |||
| 3 | 2A | 5a | H2N–Ph 6b |
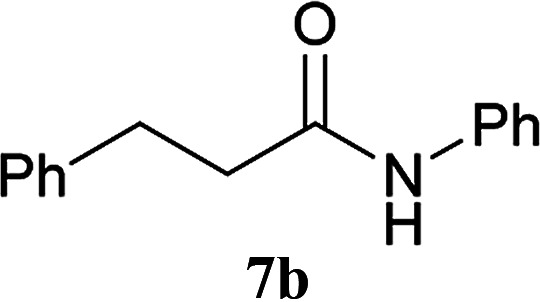
|
30 min | 91 (90) |
| 4 | 2D | 20 min | 91 | |||
| 5 | CDMT | 30 min | 65 | |||
| 6 | 2A | 5a |
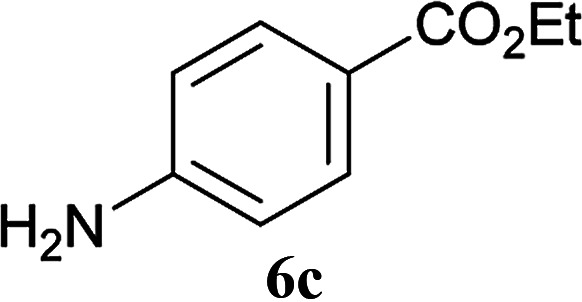
|
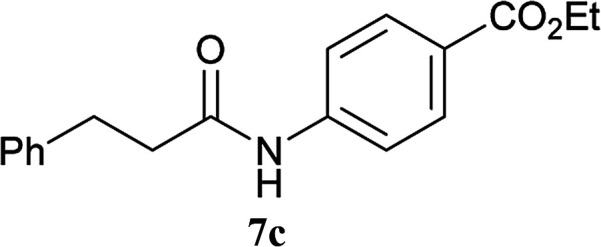
|
2 h | 92 (90) |
| 7 | 2D | 2 h | 71 | |||
| 8 | 2D | 7 h | 93 | |||
| 9 | CDMT | 2 h | 34 | |||
| 10 | 2A | iPr–CO2H 5b | H2N–iPr 6d |
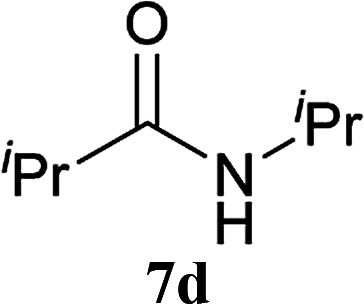
|
5 h | 91 (69) |
| 11 | CDMT | 5 h | 82 | |||
| 12 | 2A | t Bu–CO2H 5c | 6a |
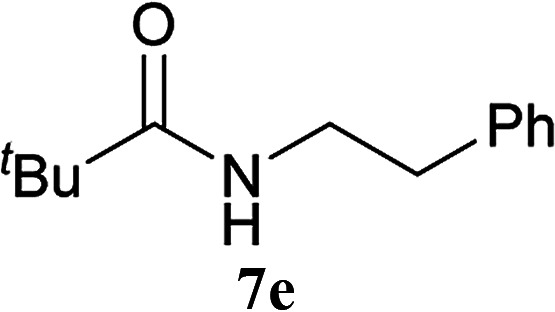
|
5 h | 78 (63) |
| 13 | CDMT | 5 h | 79 | |||
| 14b | 2A | 5c | 6d |
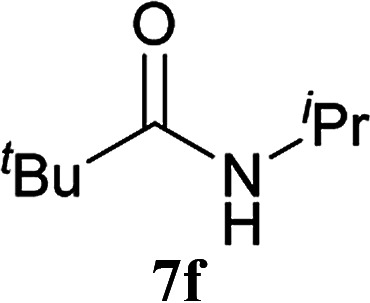
|
24 h | 77 (75) |
| 15b | CDMT | 24 h | 84 | |||
| 16b | 2A | 5c | 6b |
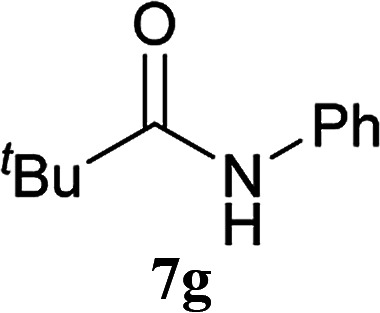
|
8 h | 69 (60) |
| 17b | CDMT | 8 h | 25 | |||
| 18 | 2A |
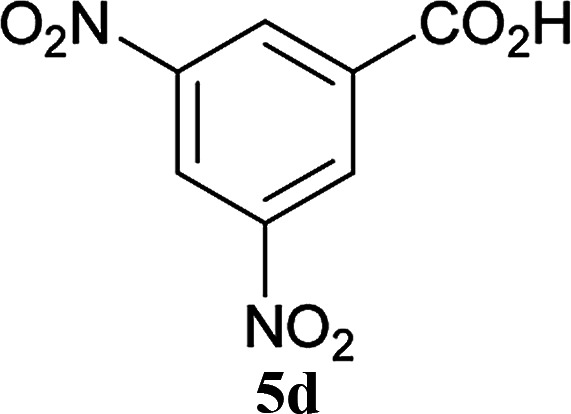
|
6a |
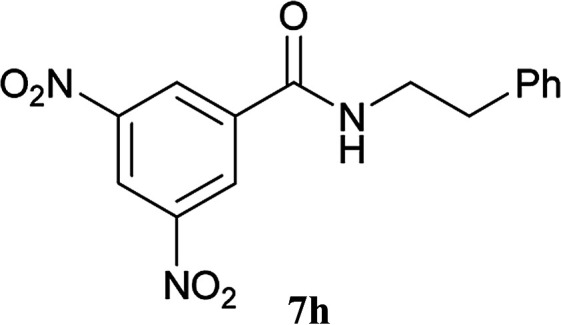
|
5 h | 96 (94c) |
| 19 | CDMT | 5 h | 86 | |||
| 20d | 2A | Boc-Leu-OH 5e | H-Phe-OMe 6e | Boc-Leu-Phe-OMe 7i | 1 h | 94 (90) |
NMR yields. Isolated yields are given in the parentheses.
Reaction time of the first step was 15 min.
The desired product was isolated in 81% yield only by extraction and recrystallization without column chromatography.
Boc-Leu-OH (1.0 eq.), 2A (1.05 eq.), NMM (1.2 eq.), H-Phe-OMe·HCl (1.2 eq.), and EtNiPr2 (1.2 eq.) were used.
