Figure 5.
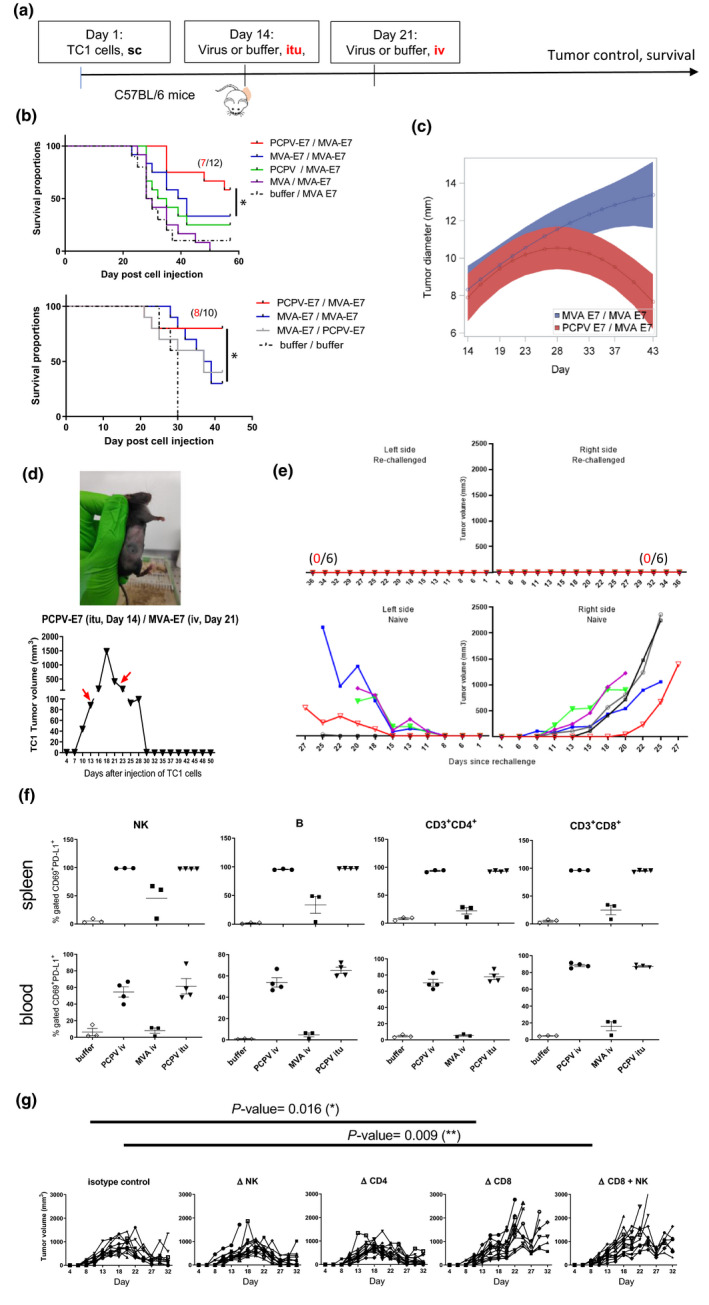
Specific antigen vaccination in the syngeneic TC1 model. (a) C57BL/6 mice (12 mice per group) were subcutaneously injected with tumor cells (5 × 105 cells). When tumors became palpable at day 14, 5 × 106 pfu of either MVA‐E7, PCPV‐E7, empty MVA or PCPV was injected into the growing tumors. One time, 106 pfu of either MVA‐E7 or PCPV‐E7 was used for the second vaccination by the intravenous route at day 21. (b) Survival data from two independent experiments are shown. Treatment effect on oversurvival was analysed using a Cox regression model for each experiment to produce a meta‐analysis. Hazard ratios and associated standard error were estimated, and meta‐analysis was performed using a fixed effect model by weighting estimation with the inverse variance. A mixed model with the treatment group, day, experiment and interactions of these terms including quadratic effect of Day was built with pooled data to estimate the differences between groups over time. The comparison between heterologous prime boost with PCPV‐E7 / MVA‐E7 vs MVA‐E7 / MVA‐E7 showed a significant difference: the P‐value was 0.0052. (c) Evolution of tumor diameter for PCPV‐E7 itu/MVA‐E7 iv (red) versus MVA‐E7 itu/MVA‐E7 iv (blue), analysed with mixed model for interaction effect day² × group, the P‐value was 0.001. (d) Example of one mouse having completely rejected a TC1 tumor after heterologous prime‐boost treatment: PCPV‐E7 was injected intratumorally at day 14 into a tumor of 90 mm3, the volume of the tumor continued to grow to 1500 mm3 until day 18, then started to regress. MVA‐E7 was injected intravenously at day 21. At day 30, tumor had completely disappeared. (e) Mice, having completely rejected TC1 tumors after heterologous prime‐boost treatment with PCPV‐E7 and MVA‐E7, were challenged with TC1 tumor cells (5 × 105), injected in both flanks. Tumor growth was monitored over 36 days (6 mice per group; upper panel: rechallenged mice, lower panel: control mice). (f) Activation status of immune cells in spleen and blood: Three or four TC1 tumor‐bearing mice were injected intravenously with 1 × 106 pfu of either MVA‐E7 or PCPV‐E7, or intratumorally with 5 × 106 pfu of PCPV‐E7. The next day, splenocytes and blood cells were analysed by flow cytometry. The percentages of CD69 and PD‐L1‐double‐positive NK, B and CD4+ or CD8+ T cells were measured. Shown are individual results per mouse and mean ± SEM per group. (g) Effect of depletion of CD8, CD4 or NK on TC1 tumor growth in mice treated with PCPV‐E7 intratumorally / and MVA‐E7 intravenously (10 mice per group). 200 µg of anti‐CD4+ (clone GK1.5) and anti‐CD8+ antibodies (clone 53‐6.7), or the isotype control for CD8 (clone 2A3), was injected intraperitoneally at days 12, 13, 20 and 28 after injection of TC1 cells. Treatment with 150 µg of the NK‐depleting antibody NK1.1 (clone PK136) was administered intraperitoneally at days 11, 13 and 15, and then twice per week. Tumor diameter evolution in individual mice is shown.
