Figure 2. Extensive disruption of developing compartments of the primate lung after LPS-induced inflammation leading to alveolar simplification.
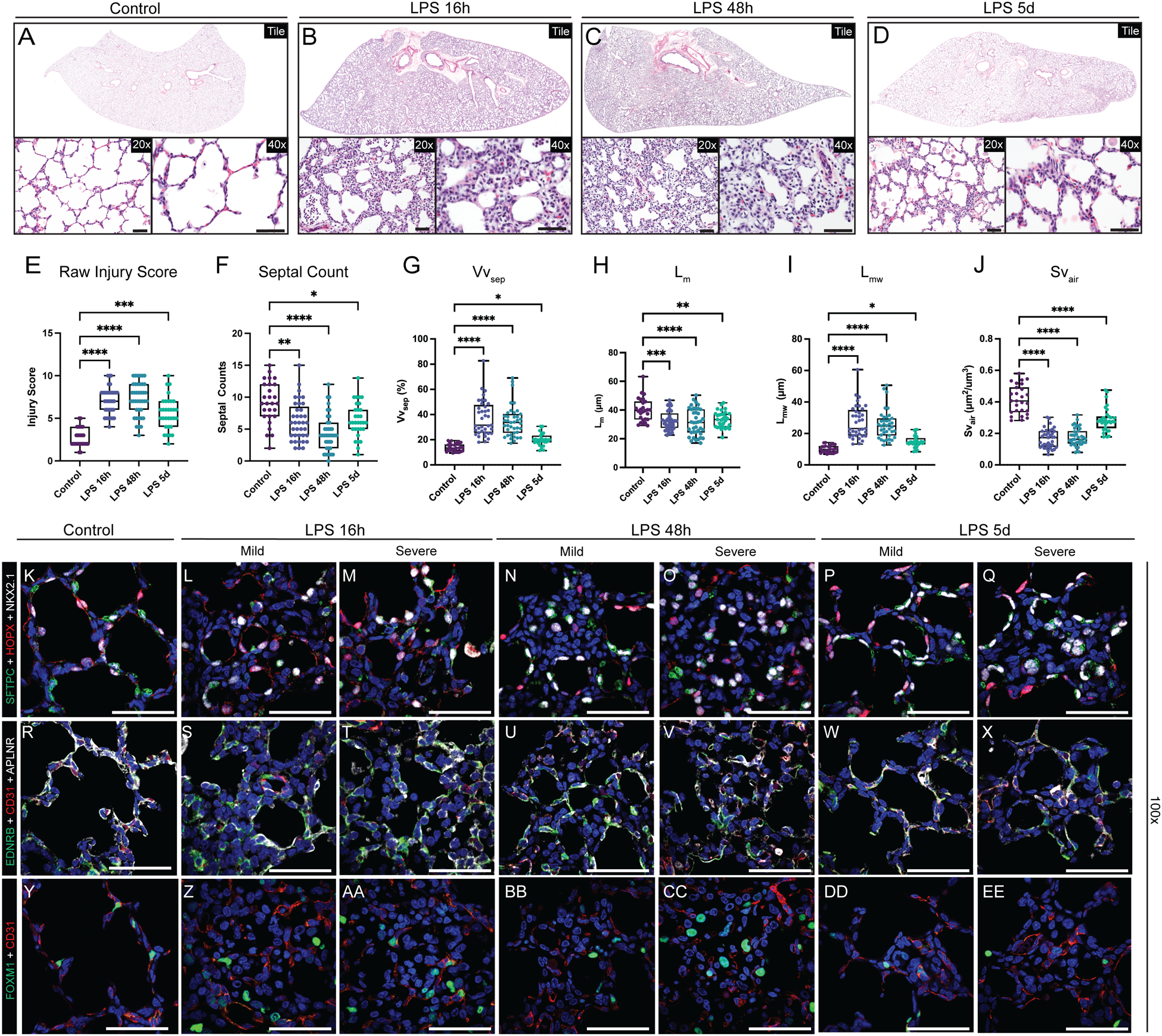
A-D) Histological analysis of LPS-induced lung injury at 16h, 48h, and 5d post-LPS. (E-J) Quantification of GD130 controls, LPS 16h, LPS 48h, LPS 5d. (E) Lung injury score calculated by ATS criteria (range = 0–10). (E) Counts of alveolar septa per field. G-J) Lung morphometry (VVsep [volume density of alveolar septa], Lm [mean linear intercept of air spaces], Lmw [mean transsectional wall length], and SVair [surface area density of air spaces]) demonstrating alveolar simplification. K-EE) Evaluation of epithelial (K-Q), differentiated capillary endothelial (R-X), and endothelial progenitor (Y-EE) populations after LPS-induced lung injury. Severe injury observed at 16h and 48h, with mild morphological improvement by 5d post-LPS. (K-Q) Destruction of organized alveolar epithelium, specifically loss of SFTPC+ AT2 cells (green) and severe damage to/disorganization of HOPX+ AT1 cells (red), with the most severe injury noted at 16h and 48h. (R-X) Loss of EDNRB+ alveolar capillaries (green) and disorganization of APLNR+ general capillaries (white) following LPS injury. (Y-EE) Nearly all FOXM1+ (green) cells lack expression of endothelial CD31, signifying loss of the CD31+/FOXM1+ proliferative endothelial progenitors. * = p <0.05, ** = p <0.01, *** = p <0.001, **** = p < 0.0001 by Kruskal-Wallace test. Scale bars = 50 μm.
