Fig. 1.
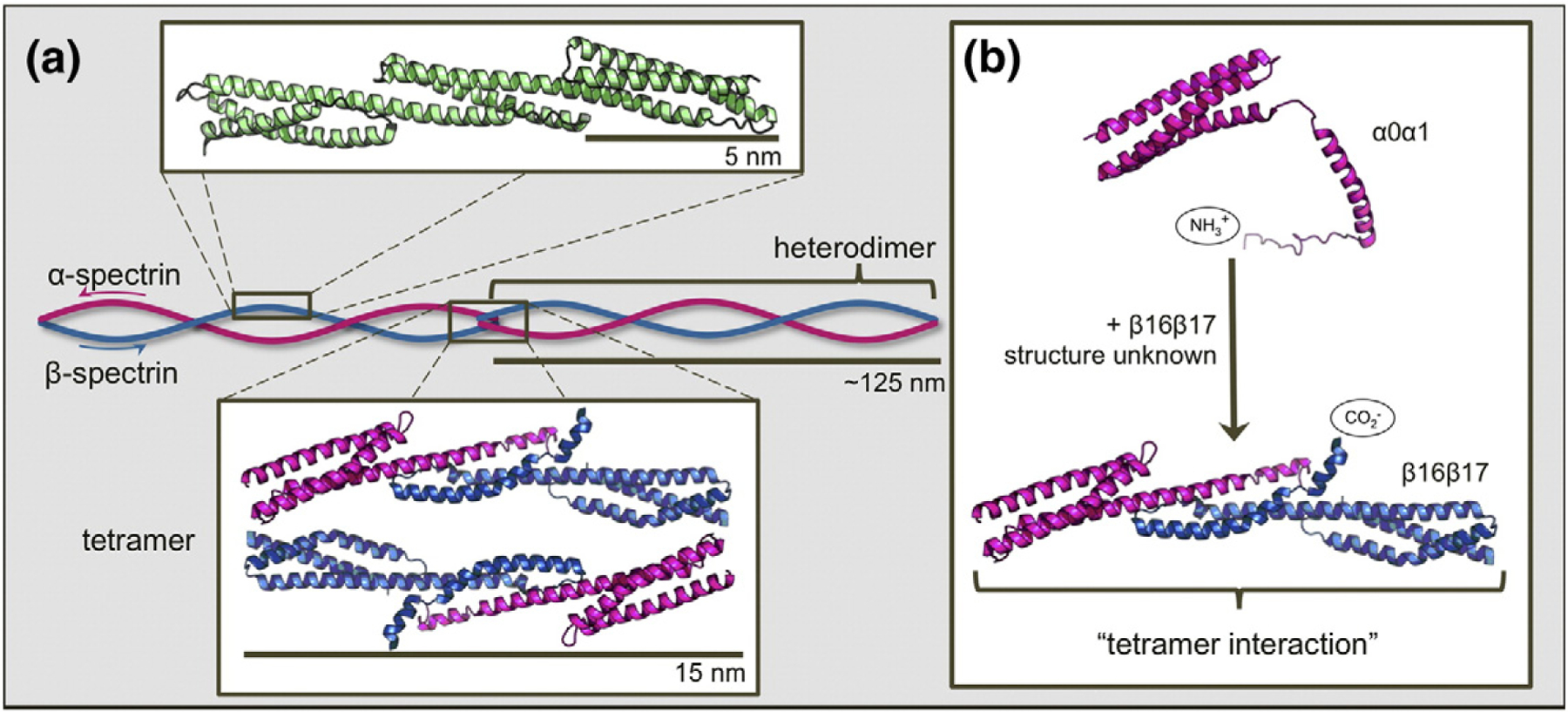
Schematic depiction of the structure and assembly of erythrocyte spectrin. (a) Three contiguous spectrin domains (green, chicken brain α-spectrin domains R15, R16, and R17; PDB ID: 1U4Q) show the three-helix-bundle structure characteristic of tandem spectrin domains. A continuous helix connects the C-helix of one domain to the A-helix of the following domain. Subunits α-spectrin and β-spectrin (pink and blue, respectively) associate laterally to form heterodimers. Where two heterodimers meet “head on”, the C-terminal tail of β-spectrin binds and folds with the N-terminal tail of α-spectrin of the opposing heterodimer to form a new, non-covalent three-helix bundle. This is termed the “tetramerization domain” (PDB ID: 3LBX). (b) Truncated constructs α0α1 (PDB ID: 1OWA) and β16β17 are employed to facilitate study of the tetramer domain as a dimerization process. Structures prepared using PyMOL (DeLano Scientific).
