Abstract
Graphene and graphene-based materials have the ability to induce stem cells to differentiate into neurons, which is necessary to overcome the current problems faced in the clinical treatment of spinal cord injury. This review summarizes the advantages of graphene and graphene-based materials (in particular, composite materials) in axonal repair after spinal cord injury. These materials have good histocompatibility, and mechanical and adsorption properties that can be targeted to improve the environment of axonal regeneration. They also have good conductivity, which allows them to make full use of electrical nerve signal stimulation in spinal cord tissue to promote axonal regeneration. Furthermore, they can be used as carriers of seed cells, trophic factors, and drugs in nerve tissue engineering scaffolds to provide a basis for constructing a local microenvironment after spinal cord injury. However, to achieve clinical adoption of graphene and graphene-based materials for the repair of spinal cord injury, further research is needed to reduce their toxicity.
Key Words: axonal regeneration, graphene, graphene oxide, nerve axon regeneration, reduced graphene oxide, spinal cord contusions, spinal cord injury, spinal cord trauma
Introduction
Spinal cord injury (SCI) is a severe trauma of the central nervous system that results in a high rate of disability (Slusarczyk et al., 2019) through motor, sensory, and sphincter dysfunctions, dystonia, and pathological reflexes. Considering the increasing incidence of car accidents and fall-related injuries (Hamid et al., 2018), the incidence of SCI is also increasing annually. SCI can be divided into two stages – primary injury and secondary injury (Manchikanti et al., 2009; Ghatas et al., 2021). Primary injury is mechanical injury caused by the trauma. This leads to SCI via external factors, such as compression, dislocation, and other mechanical forces that damage intramedullary cells, destroy axons, and cause Wallerian degeneration (Singh et al., 2014; Vismara et al., 2017). Secondary injury is caused by a series of subsequent pathological reactions, which lead to neuropathy. This further harms the nervous system and leads to irreversible injury. Secondary injury is the most important obstacle affecting nerve regeneration and late functional repair (Singh et al., 2014; Vismara et al., 2017).
Surgical decompression and high-dose corticosteroid pulse therapy are mainly used in the immediate clinical treatment of SCI. However, these treatments only alleviate the primary symptoms and reduce secondary damage, without establishing connections between the axons at the injured site to restore their physiological function (Rouanet et al., 2017; Vismara et al., 2017). In addition, stem cell transplantation,neurotrophin delivery, and implantable biomaterials are being developed to promote nerve repair after SCI. The purpose of these treatments is to delay the process of injury, promote nerve axonal regeneration, remove growth inhibitory factors, repair damaged myelin sheath, restore normal transmission of nerve stimulation signals, and restore the function of the corresponding part of the human body (Vaquero et al., 2017; Katoh et al., 2019; Wang et al., 2019b). Although the effectiveness of these treatments has been confirmed by clinical studies, issues remain in terms of their safety (Chung et al., 2010; Mortazavi et al., 2016; Kalsi et al., 2017). For example, in stem cell transplantation, stem cells secrete a variety of cytokines and can be induced to differentiate into neurons and other cells to promote nerve regeneration (Iyer et al., 2017; Robinson et al., 2017); however, transplantation of bone marrow mesenchymal stem cells has been shown to lead to tumorigenesis (Kobayashi et al., 2012). Using cytokines secreted by cells derived from macrophages can improve the local microenvironment of SCI and promote the repair of SCI; however, this does not prevent the development of SCI (Kong et al., 2018; Zhang et al., 2021).
The treatment of SCI with implantable biomaterials is one of the most important research areas in the field of SCI repair. These biomaterials can be a single material, an implant that carries a nerve regeneration drug, or a biological tissue engineering scaffold combined with seed cells for tissue construction. The biomaterials implanted at the site of SCI can build a suitable three-dimensional space for the growth of cells and guide the extension of neuronal axons, which is beneficial to the establishment of functional connections of regenerated axons. Graphene and graphene-based materials have better mechanical properties than more mature biological scaffold materials (e.g., humanoid collagen, chitosan, various gels, polylactic acid, and polyethylene glycol). These properties result in a high swelling rate of graphene-based composite scaffolds; reduce the possibility of secondary injury; and increase the affinity between the scaffold surface and the loaded cells. Moreover, the good conductivity of these biomaterials allows them to effectively conduct nerve electrical stimulation, thereby accurately guiding the formation of axons, shortening the repair cycle, and improving the rate of axon repair. Furthermore, because graphene can induce embryonic stem cells to differentiate into neurons, can induce the formation of human neuron synapses, and has excellent drug carrier properties, it can play an important role in overcoming secondary SCI (Min et al., 2017; Saravanan et al., 2018; Agarwal et al., 2020; Dominguez-Bajo et al., 2020; Usmani et al., 2020; Yang et al., 2021).
This review summarizes the advantages of these materials in the treatment of SCI (Figure 1). We discuss their potential in overcoming the difficulties in the treatment of SCI and provide a reference for researchers to perform further investigation.
Figure 1.
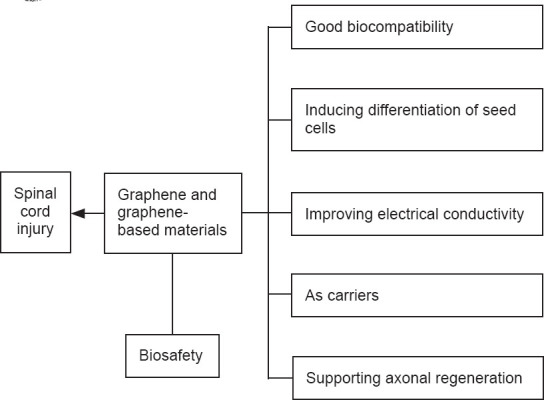
Advantages of graphene and graphene-based materials in solving the key problems in the treatment of spinal cord injury.
Search Strategy
We performed an online search of PubMed and Web of Science to retrieve related articles using the search terms “spinal cord injury” and “graphene” with the time range from inception to June 2021. A total of 86 related articles were retrieved according to the following inclusion and exclusion criteria in this narrative review. Inclusion criteria: (1) basic and clinical studies of graphene and graphene-based materials involved in the repair of spinal cord injury; (2) basic studies on the effects of graphene and graphene-based materials on nerve cells. Two researchers (SXW and YBL) independently read and screened the articles by reading the titles and abstracts and then combined the screening results. In the event of disagreement between the two researchers on the inclusion of a particular article, XXW was consulted to arrive at a decision. Any articles unrelated to SCI or axon regeneration and duplicated studies were excluded.
The search formula used in the PubMed database was: ((“spinal injury”[Title/Abstract] OR “Spinal Cord Trauma”[Title/Abstract] OR “Spinal Cord Injury”[Title/Abstract] OR “Spinal Cord Contusions”[Title/Abstract]) OR (“Spinal Cord Injuries”[Mesh])) AND ((Graphene[Title/Abstract] OR rGO[Title/Abstract] OR CNTs[Title/Abstract] OR “graphene oxide”[Title/Abstract] OR “reduced graphene oxide”[Title/Abstract]) OR ((“Graphite”[Mesh]) OR “graphene oxide” [Supplementary Concept])).
Graphene and Graphene-Based Materials
Overview of graphene and graphene-based materials
Graphene is a two-dimensional carbon nanomaterial with a hexagonal honeycomb lattice composed of carbon atoms with sp²-hybridized orbitals. Graphene has the characteristics of high strength, high flexibility and bendability, super-hydrophobicity, super-lipophilicity, and good thermal conductivity (Luo et al., 2020; Kim et al., 2021; Liu et al., 2021). Driven by increasing demand for various applications, different derivatives of this material have been fabricated (Table 1). The double-bonded structure of graphene can attach to specific functional groups or molecules through addition reactions (Zhang et al., 2017a; White et al., 2018; Bao et al., 2019; Li et al., 2020). Graphene oxide (GO) is an intermediate for the preparation of graphene. The oxygen-containing functional groups of graphene oxide make it hydrophilic and more widely used than graphene itself (Narayanan et al., 2020). Reduced graphene oxide (rGO) has a large specific surface area owing to the small number of oxygen-containing groups on its surface, giving it a sufficiently high conductivity to promote stem cell growth (Bianco et al., 2013). In contrast with GO, rGO can reduce the water dispersion of the composite material and improve the stability of stents (Zhou et al., 2019). A high electrocatalytic activity of graphene can be achieved by doping with nitrogen (Groves et al., 2009). Moreover, functionalized nitrogen-doped graphene quantum dots can be used as a photodynamic therapy (Kuo et al., 2020). Finally, graphane, which is formed upon the addition of graphene with hydrogen, shows semiconductor properties (Sofo et al., 2007), and has been studied in the repair of nerve axons (Figure 2).
Table 1.
Overview of graphene and graphene-based materials
| Type | Physicochemical properties | Main applications |
|---|---|---|
| Graphene | High electrical conductivity, high thermal conductivity, high mechanical strength and good optical properties | -Electrochemical biosensor |
| -Antibacterial | ||
| GO | High specific surface energy, good hydrophilicity and mechanical strength properties | -Promote the adhesion and proliferation of stem cells; |
| -Promote osteogenic differentiation of stem cells; | ||
| -Promote stem cells to differentiate into neurons; | ||
| -Optical biosensor; | ||
| -Drug carrier; | ||
| -Gene vector; | ||
| -Photodynamic therapy; | ||
| -Photothermal therapy; | ||
| -Biological imaging; | ||
| -Antibacterial | ||
| rGO | Good optical properties | -Promote the adhesion and proliferation of stem cells; |
| -Promote stem cells to differentiate into neurons; | ||
| -Photothermal therapy; | ||
| -Photodynamic therapy; | ||
| -Biological imaging; | ||
| -Antibacterial | ||
| Nitrogen -doped graphene | Good biocompatibility and strong electrocatalysis ability | -Biosensor; |
| -Photodynamic therapy; | ||
| -Antibacterial | ||
| Graphane | Semiconductor properties | Biosensor |
Figure 2.
Advantages of graphene and graphene-based materials in solving the key difficulties in the treatment of spinal cord injury (SCI, 2009–2021).
PLGA: Poly(lactic-co-glycolic acid).
Biomedical applications of graphene and graphene-based materials
In biomedicine, graphene is generally functionalized with different active groups (e.g. epoxy, hydroxyl, and carboxyl groups) on its surface to tailor its properties to the desired application (Georgakilas et al., 2016). Graphene-based materials can also be loaded with a drug (Liu et al., 2013; Wang et al., 2014; Zhou et al., 2014), and their size can be controlled to enable them to cross the blood-brain barrier. Rich “π” bonds on the surface and hydrogen bond binding sites can also be used to control drug release (Su et al., 2013). In addition to carrying drugs, these materials can be used for gene delivery to improve the efficacy of gene therapy (Malmsten et al., 2013; Imani et al., 2015). Furthermore, the discovery of the antimicrobial activity of graphene and graphene-based materials provides a promising solution to the problem of antimicrobial drug resistance (Kurantowicz et al., 2015; Lukowiak et al., 2016; Lima-Sousa et al., 2020).
Because of their excellent optical and electrical properties, graphene-based materials have attracted considerable attention for application as electrochemical sensors and biosensors, and in biological imaging. For example, exfoliated graphite nanosheets are widely used for blood glucose measurement, giving much higher detection sensitivity than the traditional method based on carbon nanotubes (Lu et al., 2008; Shi et al., 2015). Graphene quantum dots have excellent photoluminescence properties because of the boundary effect and quantum confinement effect. For example, Qu et al. (2015) synthesized nitrogen-doped graphene quantum dots capable of emitting a variety of colors (blue, green, and yellow). These quantum dots had low cytotoxicity and good biocompatibility, making them attractive as biological imaging agents. Such materials also have great potential in photochemical therapy of tumor replacement therapy (Yang et al., 2012; Chen et al., 2016).
Because of its excellent mechanical properties, graphene is often combined with different biomaterials to make scaffolds for bone tissue regeneration and stem cell differentiation. For example, Ignat et al. (2019) used GO scaffolds to induce cell differentiation and found that higher scaffold concentrations promoted osteogenic differentiation to greater extents. These findings were consistent with those of Elkhenany et al. (2015). In another study, Schwann cells were shown to have a significant proliferative effect on targeted aminolyzed poly-L-lactide nanofiber scaffolds coated with GO nanoparticles, highlighting the potential of this material in nerve regeneration (Zhang et al., 2016). Finally, graphene substrates have also been shown to promote nerve differentiation (Hong et al., 2014), and graphene and graphene-based materials have been used as stents implanted into the injured spinal cord to initiate a beneficial tissue response (Domínguez-Bajo et al., 2017).
Physicochemical Properties and Advantages of Graphene and Graphene-Based Materials in Axonal Repair after Spinal Cord Injury
Biomaterials have been increasingly used in the treatment of SCI, with increasing attention paid to graphene and graphene-based materials because of their suitability for axonal repair after SCI (Table 2). There is also an increasing number of in vitro cytological studies addressing the effects of graphene and graphene-based materials on nerve cells (Table 3). Graphene and graphene-based materials are used in the treatment of SCI because of their good biocompatibility, neuronal induced orientation, adsorption, electrical conductivity, and flexibility.
Table 2.
A summary of animal model experiments (in vivo) on graphene and graphene-based materials in the repair of spinal cord injury
| Reference | Animals | Material intervention mode | Modeling method | Material intervention time | Detection method | Main results | Significance |
|---|---|---|---|---|---|---|---|
| Kolarcik et al., 2015 | Adult male Sprague-Dawley rats | Conductive polymer poly(3,4-ethylenedioxythiophene) (PEDOT) and multi-wall CNTs were coated on the electrode surface and doped with the anti-inflammatory drug dexamethasone | A unilateral laminectomy was performed to expose the left side of the dorsal root ganglion between L5 and L6 | 14 d | (1) Confocal fluorescent microscopy; | Significantly less neuronal death/damage was observed with coated electrodes and the inflammatory was also reduced. | This study was the first to report the utility of these coatings in stimulation applications. |
| (2) Immunofluorescence | |||||||
| López-Dolado et al., 2015 | Aged adult male Wistar rats | 3D flexible and porous scaffolds composed of partially rGO | A right lateral hemisection of approximately 8 mm3 (2 mm × 2 mm × 2 mm) at the C6 segment, rostral to the bulk of triceps brachii motoneurons | 10 d | (1) Histological examination; (2) Immunofluorescence | These structures facilitated regaining tissue integrity after SCI as early as 10 d and prevent the extension of the lesion. It had no local and systemic toxic responses. | This study was the first to implant 3D porous and flexible rGO scaffolds at the injured rat spinal cord. |
| López-Dolado et al., 2016 | Adult male Wistar rats | 3D scaffolds composed of partially rGO | A right lateral hemisection of approximately 8 mm3 (2 mm × 2 mm × 2 mm) at C6, rostral to the bulk of triceps brachii motoneurons | 30 d | (1) Histological examination; | The scaffolds in injury stabilization and sealing, moreover, rGO scaffolds supported angiogenesis. | This study investigated for the first time chronic tissue responses to 3D scaffolds composed of partially rGO when implanted in the injured rat spinal cord. |
| (2) Immunofluorescence; | |||||||
| (3) Transmission electron microscopy | |||||||
| Palejwala et al., 2016 | Wistar rats (19 males and 1 female) | Graphene nanoscaffolds were prepared by the mild chemical reduction of GO | Hemispinal cord transection at approximately the T2 level | 3 mon | (1) Electron microscopic; | The graphene nanoscaffolds adhered well to the spinal cord tissue. | Graphene is a nanomaterial that is biocompatible with neurons and may have significant biomedical application. |
| (2) Histological examination; | |||||||
| (3) Immunofluorescence | |||||||
| González-Mayorga et al., 2017 | Adult male Wistar rats | rGO microfibers as substrates for promoting nerve growth | A right lateral hemisection of approximately 8 mm3 (incomplete lesion) at C6, rostral to the bulk of triceps brachii motoneurons. | 10 d | (1) Scanning electron microscope; | In vivo studies reveal the feasible implantation of these rGO microfibers as a guidance platform in the injured rat spinal cord, without evident signs of subacute local toxicity. | These positive findings boost further investigation for enhancing repair in the damaged central neural tissue including the injured spinal cord. |
| (2) Transmission electron microscopy; | |||||||
| (3) Immunofluorescence | |||||||
| Domínguez-Bajo et al., 2019 | Adult male rats | 3D randomly porous foams have been prepared in mechanical compliance with neural cells and tissues (Young’s modulus of 1.3 ± 1.0 kPa) as demonstrated by atomic force microscopy techniques applied ex vivo. | A cervical unilateral hemisection at the right C6, rostral to the bulk of triceps brachii motoneurons | 4 mon | (1) Transmission electron microscopy; | The scaffolds significantly reduced perilesional damage and caused no compressive damage in the contralateral hemicord and rostral/caudal regions. It also does not either alter the rat spontaneous behavior or induce toxicity in major organs. | This study suggests hints of rGO sheets dissociation and eventual degradation at the injured spinal cord for the first time. |
| (2) Magnetic resonance imaging; | |||||||
| (3) Atomic force microscopy; | |||||||
| (4) Immunofluorescence; | |||||||
| (5) Histological examination; | |||||||
| (6) Behavioral tests | |||||||
| Pan et al., 2019 | Female Sprague-Dawley rats | IGF-1 and BDNF were successfully immobilized on biodegradable GO-incorporated PLGA electrospun nanofibres. | T9 spinal cord hemisection rat model | 4 wk | (1) Immunofluorescence; | Local delivery of IGF-1 and BDNF immobilized to PLGA/GO nanofibres significantly improved functional locomotor recovery, reduced cavity formation and increased the number of neurons at the injury site. | This study indicated that PLGA/GO is an effective carrier for IGF-1 and BDNF delivery. |
| (2) Motor function detection; | |||||||
| (3) Histology observations; | |||||||
| (4) The BBB locomotor rating scale; | |||||||
| (5) Motor evoked potential detection | |||||||
| Domínguez-Bajo et al., 2020 | Adult male rats | rGO materials in the shape of microfibers | A right hemisection at C6 cervical level, rostral to the bulk of triceps brachii motoneurons | 10 d | (1) Behavioural tests; | These findings outline the potential of rGO-MF-based scaffolds to promote regenerative features at the injured spinal cord such as axonal and vascular growth. | In this work, the regenerative potential of rGO-MFs when chronically interfaced with a cervical spinal cord injury was investigated for the first time. |
| (2) Immunofluorescence | |||||||
| Yang et al., 2021 | Female Sprague-Dawley rats | A conductive GO composited chitosan scaffold was fabricated by genipin crosslinking and lyophilization. | The lamina of the thoracic vertebrae T8–T10 were exposed. The spinal cord was exposed and approximately 2 mm of the spinal cord tissue at the T9 level was completely removed under an operating microscope | 10 wk | (1) The BBB locomotor rating scale; | GO could have a positive role in the recovery of neurological function after SCI by promoting the degradation of the scaffold, adhesion, and migration of nerve cells to the scaffold. | The scaffold can promote the repair of damaged nerve tissue. |
| (2) Electrophysiologic recording; | |||||||
| (3) Histological analysis; | |||||||
| (4) Immunofluorescence |
BBB: Basso, Beattie, and Bresnahan; BDNF: brain-derived neurotrophic factor; CNTs: Carbon nanotubes; IGF-1: Insulin-like growth factor 1; min: minute; PLGA: poly (lactic-co-glycolic acid); rGO-MFs: reduced graphene oxide materials in the shape of microfibers; SCI: spinal cord injury.
Table 3.
Summary of in vitro cytological studies on the effects of graphene and graphene-based materials on nerve cells
| Year of publication | Cell type | Nerve cell sources | Culture method | Detection method | Main results | Significance |
|---|---|---|---|---|---|---|
| Chen et al., 2012 | Human embryonic stem cells | Wicell (Madison, WI, USA; passages 32 to 55) | A neuron induction medium consisting of F12/DMEM, N2 supplement, and FGF2 (20 ng/mL) | Immunocytochemistry and fluorescence measurement; scanning electron microscopy | The results demonstrated that cells on silk-CNT scaffolds have a higher β-III tubulin and nestin expression, suggesting augmented neuronal differentiation. | The silk-CNT composite scaffolds developed here can serve as efficient supporting matrices for stem cell-derived neuronal transplants. |
| Solanki et al., 2013 | Neural stem cells | Unclear | Proliferated in culture media containing basic fi broblast growth factor (bFGF, 20 ng/mL) and epidermal growth factor (EGF, 20 ng/mL) | Quantitative RT-PCR; scanning electron microscopy; immunostaining | This work has demonstrated that the graphene-nanoparticle hybrid structures lead to enhanced neuronal differentiation of hNSCs along with significant axonal alignment. | The hybrid nanostructures have tremendous implications for the potential use of GO as an ECM component especially in the field of neurobiology. |
| Tu et al., 2013 | Primary rat hippocampal neurons | Prepared from postnatal Sprague-Dawley rat pups (aged 1 to 3 d) | The DMEM medium supplemented with 10% fetal calf serum, 0.5 mM L-glutamine, 0.03 mM glucose, 100 U/mL penicillin, and 0.1 mg/mL streptomycin | Scanning electron microscopy; Immunocytochemistry; western blotting | These biomimetic choline-like GO composites can significantly boost neurite sprouting and outgrowth. | All results demonstrate the potential of DMAEMA- and MPC-modified GO composites as biomimetic materials for neural interfacing. |
| González-Mayorga et al., 2017 | Embryonic neural progenitor cells | Obtained from cerebral cortices of E18 Wistar rat embryos | 500 μL of complete neurobasal media containing B-27 supplement (2%), streptomycin (100 U/mL), penicillin (100 U/mL), and L-glutamine (1 mM) | Scanning electron microscopy; confocal laser scanning microscopy; transmission electron microscopy | These microfibers behave as supportive substrates of highly interconnected cultures composed of neurons and glial cells for up to 21 days, and the colonization by meningeal fibroblasts is dramatically hindered by N-cadherin coating. | These positive findings boost further investigation at longer implantation time and enhancing repair in the damaged central neural tissue including the injured spinal cord. |
| Min et al., 2017 | Human neuroblastoma cells | American type culture collection (Manassas, VA, USA) | The DMEM medium supplemented with 10% heat-activated fetal bovine serum and 1% antibiotics (penicillin and streptomycin) | Fluorescence imaging; confocal imaging; Raman spectroscopy | The study developed a novel MF-driven GO hybrid pattern that was highly effective for controlling synaptogenesis. | This work provides treatment and modeling of brain diseases and spinal cord injuries. |
| Serrano et al., 2018 | Embryonic neural progenitor cells | Obtained from cerebral cortices of E18 Wistar rat embryos | 500 μL of complete neurobasal media containing B-27 supplement (2%), streptomycin (100 U/mL), penicillin (100 U/mL), and L-glutamine (1 mM) | Confocal laser scanning microscopy; inflammatory cytokine detection; flow cytometry | The capacity of rGO microfibers to inhibit the proliferation of RAW-264.7 macrophages, without affecting their viability and cell cycle profiles. | These findings encourage further investigation of these microfibers as attractive biomaterials to interact with neural cells. |
| Pan et al., 2019 | Neural stem cells | Isolated from the cerebral cortex of embryonic mice (E11.5) | The growth media contained neurobasal media, B27 neural supplement, 100 ng/mL penicillin-streptomycin, 20 ng/mL epidermal growth factor and 20 ng/mL basic fibroblast growth factor. | MTT assay; immunofluorescence; quantitative real-time PCR analysis | PLGA/GO nanofibres loaded with IGF-1 and BDNF not only protected NSCs from oxidative stress induced by H2O2 but also enhanced NSC proliferation and neuronal differentiation in vitro. | The study indicated that immobilization of IGF-1 and BDNF onto PLGA/GO nanofibres has a great potential as a nerve implant for spinal cord injury applications. |
| Domínguez-Bajo et al., 2020 | Embryonic neural progenitor cells | Isolated from cerebral cortices of gravid Wistar rats | Samples were covered with 500 L of complete neurobasal media containing B-27 supplement (2%), streptomycin (100 U/mL), penicillin (100 U/mL), and glutamine (1 mM). | Immunofluorescence studies by confocal laser scanning microscopy | This work confirmed the capacity of rGO-MFs to support the growth of ENPCs in vitro. | In this work, the regenerative potential of rGO-MFs when chronically interfaced with a cervical spinal cord injury was investigated for the first time. |
| Li et al., 2020 | Primary rat Schwann cells | The spinal nerves of Sprague-Dawley rats (newborn to postnatal d 4–5) | The cells were cultured with laminin coated culture plate or bottle in DMEM/F12 containing 15% FBS and 1% penicillin-streptomycin. | Scanning electron microscopy; western blotting; MTT assay; immunofluorescence | The results of cell experiments indicated the better adhesion and higher expression of neural proteins in rat Schwann cells (RSCs) on PDA/CGO/PPy-PLLA films. | These results indicated that PDA/CGO/PPy-PLLA films were promising to be applied for the ES therapy of peripheral nerve repair in vivo. |
CGO: Carboxylic graphene oxide; CNTs: carbon nanotubes; DMAEMA: dimethylaminoethyl methacrylate; DMEM/F12: Dulbecco’s modified Eagle media: nutrient mixture F-12; ECM: cell-extracellular matrix; EGF: epidermal growth factor; ENPCs: embryonic neural progenitor cells; FBS: fetal bovine serum; FGF: fibroblast growth factor; MF: magnetic force; MPC: 2-methacryloyloxyethyl phosphorylcholine; NSCs: neural stem cells; PDA: polydopamine; PLLA: ply-l-lactic acid; PPy: polypyrrole; TH: L-theanine.
The biological compatibility of graphene and graphene-based materials with neurons and other cells is the basis of their application in biomedicine, because it allows cells to adhere well and grow stably. While exploring the biocompatibility of different types of carbon-based nanomaterials, including graphene and graphene-based materials, Ryoo et al. (2010) found that focal adhesion cells in the control group were larger and fewer in number than those in the experimental group, which indicates that the affinity of the cells to these materials was higher than the control. The cells on the carbon-based materials were more difficult to separate in the later stage of cell separation and were biocompatible. This study lay the foundation for subsequent research into the treatment of SCI. In a study of a chitosan-β-glycerophosphate thermosensitive hydrogel containing GO, the hydrogel was shown to have good biocompatibility with bone marrow mesenchymal stem cells (Saravanan et al., 2018). The results of another experiment showed that human osteoblasts and mesenchymal stem cells had good proliferation ability on graphene-based thin films (Marie et al., 2010); the authors of this study also suggested that, as an artificial nerve tissue engineering scaffold, graphene has the potential to promote axonal repair after SCI. Furthermore, Guo et al. (2017) confirmed that cell-compatible rGO microfibers not only provide a more powerful matrix for the adhesion and proliferation of neural stem cells, but also induce neural stem cells to differentiate into neurons. An anti-inflammatory effect in a co-culture of microglia and graphene foam also demonstrates the application potential for the treatment of SCI (Song et al., 2014). In addition to the aforementioned in vitro studies, there is evidence that these graphene-based composite materials are effective in the treatment of SCI in animal models (Sahni et al., 2013; Palejwala et al., 2016). These studies have confirmed the growth of connective tissue elements, blood vessels, nerve filaments, and Schwann cells around and inside the scaffold, which demonstrates the scaffold’s suitability for the growth of regenerated axons after SCI.
Advantages of graphene and graphene-based materials in inducing differentiation of seed cells
A promising strategy to promote axonal regeneration after SCI is to induce seed cell differentiation (Lee-Kubli et al., 2015). Tissue engineering scaffolds made from graphene or its derivatives construct a suitable space for nerve repair in the later stage of SCI and axon regeneration. When combined with seed cells, they also facilitate proliferation and differentiation (Silver et al., 2014; He et al., 2016). Using a novel amino-functionalized graphene crosslinked collagen-based nerve conduit, Agarwal et al. (2020) confirmed that graphene-based scaffolds, under certain conditions, can make bone marrow mesenchymal stem cells differentiate into neurons, promote neuronal cell proliferation and migration, and inhibit neuro-inflammation. GO can also induce embryonic stem cells to differentiate into neurons. For example, GO can effectively enhance the dopamine neuronal differentiation of embryonic stem cells and enhance the expression of dopamine neuron-related genes (Yang et al., 2014). Another report found that graphene can promote neural stem cells to differentiate into neurons rather than glial cells because of its unique surface properties (Guo et al., 2021). In another example, rGO microfibers were implanted into the spinal cord of injured rats and were colonized by cells, without signs of subacute local toxicity (González-Mayorga et al., 2017). Akhavan et al. (2013) used rGO nanoribbons as a photocatalyst. Under the excitation light of the corresponding wavelength, the scaffold induced more nerve differentiation than glial cell differentiation, and, at the same time, enabled neurons to grow along the grid morphology of the scaffold. Spontaneous axonal regeneration in the local area of SCI is very difficult. Thus, stem cell transplantation can cause stem cells to integrate and differentiate at the injured site, form axon structures, connect the remaining parts, and partially restore the neural system (Kumamaru et al., 2019). In a recent study, rGO promoted the regeneration of axons and blood vessels in and around the injured site, without aggravating the local inflammatory response (Dominguez-Bajo et al., 2020). Finally, Ning et al. (2011) explored the effects of a graphene matrix on synaptic development in mouse hippocampal cell culture model and found that graphene matrix could promote axonal germination and neurite growth to the greatest extent. These findings encourage the further development of graphene and graphene-based materials as nerve regeneration biomaterials for axonal therapy after SCI.
Electrical conductivity of graphene and graphene-based materials in axonal repair
Graphene and graphene-based materials have advantages over other materials in axonal regeneration. These materials can enhance signal conduction between synapses and establish or restore conduction pathways, such that nerves can conduct signals and accelerated recovery of limb motor function can be achieved. The key to functional recovery after SCI is the regeneration of axons. Differentiated and mature neurons need to transmit signals to complete a series of complex behavioral reflexes. Precise control of axon growth and synaptic connection formation are very important for repairing neural networks. Below, we provide examples of studies that have used graphene-based materials to achieve axonal regeneration.
Using a 3D scaffold made of graphene, Rauti et al. (2020) conducted electrical stimulation and accelerated stem cell differentiation in axonal repair to regulate the formation of neuronal circuits. Guo et al. (2016b) cultured mesenchymal stem cells on a poly (3,4-ethylene dioxythiophene) (PEDOT)–rGO microfiber scaffold and observed enhanced proliferation and good neural differentiation. This effect only relied on the electrical pulses generated by human walking. A GO-based patterned substrate with a layered structure was shown to promote cell adhesion and stem cell differentiation into neuron-like cells for treatment of neurodegenerative diseases (Yang et al., 2016). Another study showed that graphene can affect the bioelectric properties of the cell membrane and accelerate the maturation of neural stem cells (Guo et al., 2016a). Wang et al. (2019a) combined the electrical conductivity of graphene with electrospun nanofibers in vivo and in vitro, and confirmed that the conductive scaffold could be used for axonal regeneration. This work laid the foundation for the use of these materials in promoting repair and treatment following SCI. Using a 3D rGO fiber-scaffold, Girão et al. (2020) achieved the recovery of highly active neural networks in vitro. Their findings provide a strong rationale for future in vivo studies on spinal cord regeneration. In an earlier study, a magnetically driven GO hybridization model was used to control the connection between synapses and match the synaptic network between neurons by guiding the direction of axons (Min et al., 2017). This model has proved useful for modeling brain diseases and SCI.
A chitosan-GO scaffold was shown to greatly improve the neurological function of rats by facilitating nerve repair after SCI (Valencia et al., 2021; Yang et al., 2021). Chaejeong et al. (2011) controlled neural cell-to-cell interactions in a cell culture by non-contact electric field stimulation with a graphene electrode using a graphene-polyethylene terephthalate film electrode. Under stimulation of a weak electric field, the number of cells forming new intercellular coupling and strengthening intercellular coupling increased markedly. In a final example, Yao et al. (2008) observed the directional migration response of rat hippocampal neurons in an external electric field. This work introduced the possibility of using electrical conductivity of graphene and its derivatives in nerve axon repair after SCI.
Advantages of graphene and graphene-based materials in supporting axonal regeneration
Establishment of spatial structure, reduction of angiogenesis, and glial scar formation are the most important effects of graphene and graphene-based materials in axonal regeneration. The pores in the spatial structure established by the biomaterial scaffold enable interactions between axons and neovascularization, and allow the material to serve as a stem cell carrier. This improves the local microenvironment at the site of injury. These materials can also be loaded with neurotrophic factors and other regenerative drugs to fully contact the injured site. Below, we provide examples of angiogenesis and glial scare formation using graphene-based materials.
López-Dolado (2016) reported an rGO composite stent to support angiogenesis around a lesion. They observed the formation of rich and functional new blood vessels in the stent (in contrast with the control group, in which malformed lesions and voids were observed). They also found some regenerated neuronal axons in the proximal end of the stent, in contrast with the control group. Usmani et al. (2020) found anti-endothelial cell antibody (RecA-1)-positive cells in 3D multi-walled carbon nanotubes. The appearance of RecA-1-positive cells is a sign of vascular endothelial cell maturation, and indicates that the carbon-containing scaffolds support microangiogenesis to help repair the injury. Domínguez-Bajo et al. (2019) observed changes of the surrounding tissue after implantation of mechanically compliant rGO foam into the injured site. The authors found that this material can help the damaged spinal cord repair nerve function by promoting blood vessel remodeling and axon regeneration. Moreover, the GO foam avoided progression of SCI by eliminating scar retraction and reducing the proliferation of glia above and below the center of the SCI to the surrounding area. Chakraborty et al. (2018) added rGO to a polyvinylalcohol-carboxymethyl cellulose hydrogel to successfully promote angiogenesis and arteriogenesis.
Many studies (Windle, 1956; Kimura-Kuroda et al., 2010; Dolma and Kumar, 2021; Hart and Karimi-Abdolrezaee, 2021) have confirmed that the inflammatory response of secondary injury activates a large number of astrocytes around the injured foci. They also have confirmed that the glial scar not only produces a direct physical barrier to the regeneration of nerve injured axons, but also releases a series of factors to inhibit axonal regeneration and growth. However, Andersen et al. (2016) concluded that the formation of astroglial scars is not just the main reason for axonal regeneration after nervous system injury; it also contributes to central nervous system axonal regeneration under appropriate stimulation. The dual role of glial scars needs to be further explored.
Advantages of graphene and graphene-based materials as drug carriers
Graphene and graphene-based materials have excellent drug carrier properties and are able to carry drugs directly to the action target and maintain their effective concentration. For example, Zhang et al. (2010b) and Yang et al. (2018) reported ultrahigh drug loading capacity of doxorubicin on GO of approximately 400% and 238%, respectively. This is mainly because of the ultrahigh specific surface area of GO, which can adsorb drugs through electrostatic interactions and π–π stacking (Zhang et al., 2010b). Moreover, the rich functional groups of GO can directly (through electrostatic interactions) immobilize enzymes without the need for cross-linking agents or additional modification (Zhang et al., 2010a). Using a polylactide-glycolic acid nanofiber scaffold coated by GO and methylene blue, Wang et al. (2019c) loaded and released methylene blue from the matrix to protect and regulate the function of neural progenitor cells. Although this approach was developed for the treatment of Alzheimer’s disease, it could also be used in the recovery of nerve axons after SCI. Graphene has also been used to deliver growth factors to the damaged site, which is necessary for regeneration. For example, Pan et al. (2019) immobilized insulin-like growth factor-1 and brain-derived neurotrophic factor on biodegradable GO-PLGA electrospun nanofibers. This material protected neural stem cells from oxidative stress damage of H2O2 in vitro and promoted neural stem cell proliferation and neuronal differentiation. In an animal model of SCI, local injection promoted functional motor recovery, reduced the formation of a cavity in the injured site, and increased the number of neurons (Pan et al., 2019). As a final example, a graphene drug carrier was shown to enhance the neuroprotective effect of the drugs, reduce spinal cord edema, and improve functional recovery after SCI (Zhang et al., 2010c). Graphene is expected to become a key material for the treatment of conditions such as neuropathy.
Biosafety of Graphene and Graphene-Based Materials
Despite the otherwise ideal properties of graphene and graphene-derived materials for use in biomedicine, their toxicity needs to be further investigated. The biotoxicity of these materials is controversial (Seabra et al., 2014). For example, some research has found that rGO was more likely to cause membrane damage and oxidative stress than GO (Zhang et al., 2018), whereas other studies have reported the opposite trend (Palejwala et al., 2016; Tabish et al., 2017). The toxicity of graphene and graphene-based materials is directly related to the material size, surface functional groups, and dose (Jastrzębska et al., 2012; López-Dolado et al., 2015; Ma et al., 2015). For example, especially in long-term culture, larger GO particles show higher toxicity than smaller particles (Mendes et al., 2015). Moreover, excessive GO has been shown to produce cytotoxicity (Jing et al., 2015; Zhang et al., 2017b). At high doses, GO has also been observed to accumulate in the respiratory tract, block pulmonary vessels, and lead to dyspnea (Li et al., 2014). Although some studies suggest that there is a safe range of conditions (Sasidharan et al., 2012; Yue et al., 2012), further experiments are needed before these materials are introduced into the clinical treatment of human SCI.
The degradability of implantable biomaterials is also an important index to evaluate their safety. Graphene degrades in vivo, as shown by Schinwald et al. (2014) in a study in which mice inhaled unoxidized graphene crystals. Similarly, rGO is biodegradable (Kurapati et al., 2015, 2018). By contrast, GO is difficult to be eliminated by the lungs (Ou et al. 2016).
Summary and Future Prospects
Summary
In summary, graphene and graphene-based materials have considerable potential as scaffold materials for nerve tissue engineering that promote axon repair and regeneration. They provide a new means of overcoming the difficulties currently faced in the treatment of SCI in its late stages.
Limitations of graphene and graphene-based materials
Some studies have shown that graphene and graphene-based materials are cytotoxic, increase oxidative stress, and block pulmonary vessels. However, the biosafety of graphene and graphene-based materials is not yet clear. These safety concerns prevent such materials from being adopted in the clinical treatment of SCI. However, toxicity must be considered with respect to the dose and molecular properties. Therefore, we believe that safe dosage of graphene and graphene-based materials in the treatment of SCI is achievable. This needs to be further clarified in the future by dose gradient experiments and comparative analysis of different materials. This will pave the way to implementation in the treatment of SCI.
Conclusions
Effective treatment strategies for SCI are a great challenge in modern medicine. Graphene-based medical engineering may provide a solution to this problem. We summarize the advantages of these materials as follows: (1) They have good tissue compatibility, and do not cause serious toxicity or immune rejection after implantation in animal models; (2) They induce seed cells to differentiate into nerve cells, replacing the lost nerve cells in SCI, thus providing a cellular basis for the bridging of nerve axons; (3) The good electrical conductivity of graphene and graphene-based materials allows them to make full use of neuroelectrical signals in the spinal cord tissue; (4) These materials stimulate axonal regeneration; and (5) Composites based on graphene and graphene-based materials can be used as a matrix for loading seed cells, nutritional factors, and drugs to reconstruct the local microenvironment after SCI.
However, some studies have shown that graphene or graphene-based materials can cause cytotoxicity, aggravate oxidative stress, and block pulmonary blood vessels, and the biosafety research data of graphene and graphene-based materials have not been conclusive. These safety issues seem to hinder the application of graphene and graphene-based materials in the treatment of SCI. However, it is well known that it is against the spirit of science to discuss toxicity without considering the dose and molecular characteristics. Therefore, we believe that the safe dosage form and dosage range of graphene and graphene-based composites for the treatment of SCI should be explored by researchers in the future. Only after accurate experimental conclusions are obtained through a dose gradient study and a comparative analysis of different composite materials can the graphene and graphene-based composite materials be used in the treatment of SCI.
Footnotes
Funding: This work was supported by the Lanzhou Talent Innovation and Entrepreneurship Project, No. 2020-RC-40 (to XXW and YBL) and Cuiying Scientific Training Program for Undergraduates of Lanzhou University Second Hospital, Nos. CYXZ2020-03, CYXZ2021-01 (both to YBL).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Brotchie A, Song LP; T-Editor: Jia Y
References
- 1.Agarwal G, Kumar N, Srivastava A. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Mater Sci Eng C Mater Biol Appl. 2021;118:111518. doi: 10.1016/j.msec.2020.111518. [DOI] [PubMed] [Google Scholar]
- 2.Akhavan O, Ghaderi E. Differentiation of human neural stem cells into neural networks on graphene nanogrids. J Mater Chem B. 2013;1:6291–6301. doi: 10.1039/c3tb21085e. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao TJ, Wang ZY, Zhao Y, Wang Y, Yi XS. Long-term stably dispersed functionalized graphene oxide as an oil additive. RSC Adv. 2019;67:39230–39241. doi: 10.1039/c9ra07685a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco A, Cheng HM, Enoki T, Gogotsi Y, Hurt RH, Koratkar N, Kyotani T, Monthioux M, Park CR, Tascon J, Zhang J. All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon. 2013;65:1–6. [Google Scholar]
- 6.Chakraborty S, Ponrasu T, Chandel S, Dixit M, Muthuvijayan V. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R Soc Open Sci. 2018;5:172017. doi: 10.1098/rsos.172017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CS, Soni S, Le C, Biasca M, Farr E, Chen EY, Chin WC. Human stem cell neuronal differentiation on silk-carbon nanotube composite. Nanoscale Res Lett. 2012;7:126. doi: 10.1186/1556-276X-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YW, Su YL, Hu SH, Chen SY. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliv Rev. 2016;105:190–204. doi: 10.1016/j.addr.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Chung TW, Chang YL. Silk fibroin/chitosan-hyaluronic acid versus silk fibroin scaffolds for tissue engineering:promoting cell proliferations in vitro. J Mater Sci Mater Med. 2010;21:1343–1351. doi: 10.1007/s10856-009-3876-0. [DOI] [PubMed] [Google Scholar]
- 10.Dolma S, Kumar H. Neutrophil, extracellular matrix components, and their interlinked action in promoting secondary pathogenesis after spinal cord injury. Mol Neurobiol. 2021 doi: 10.1007/s12035-021-02443-5. doi:10.1007/s12035-021-02443-5. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez-Bajo A, González-Mayorga A, Guerrero CR, Palomares FJ, García R, López-Dolado E, Serrano MC. Myelinated axons and functional blood vessels populate mechanically compliant rGO foams in chronic cervical hemisected rats. Biomaterials. 2019;192:461–474. doi: 10.1016/j.biomaterials.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Bajo A, González-Mayorga A, López-Dolado E, Munuera C, García-Hernández M, Serrano MC. Graphene oxide microfibers promote regenerative responses after chronic implantation in the cervical injured spinal cord. ACS Biomater Sci Eng. 2020;6:2401–2414. doi: 10.1021/acsbiomaterials.0c00345. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-Bajo A, González-Mayorga A, López-Dolado E, Serrano MC. Graphene-derived materials interfacing the spinal cord:outstanding in vitro and in vivo findings. Front Syst Neurosci. 2017;11:71. doi: 10.3389/fnsys.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkhenany H, Amelse L, Lafont A, Bourdo S, Caldwell M, Neilsen N, Dervishi E, Derek O, Biris AS, Anderson D, Dhar M. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells:potential for bone tissue engineering. J Appl Toxicol. 2015;35:367–374. doi: 10.1002/jat.3024. [DOI] [PubMed] [Google Scholar]
- 15.Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, Zboril R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev. 2016;116:5464–5519. doi: 10.1021/acs.chemrev.5b00620. [DOI] [PubMed] [Google Scholar]
- 16.Ghatas MP, Khan MR, Gorgey AS. Skeletal muscle stiffness as measured by magnetic resonance elastography after chronic spinal cord injury:a cross-sectional pilot study. Neural Regen Res. 2021;16:2486–2493. doi: 10.4103/1673-5374.313060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girão AF, Sousa J, Domínguez-Bajo A, González-Mayorga A, Bdikin I, Pujades-Otero E, Casañ-Pastor N, Hortigüela MJ, Otero-Irurueta G, Completo A, Serrano MC, Marques PAAP. 3D reduced graphene oxide scaffolds with a combinatorial fibrous-porous architecture for neural tissue engineering. ACS Appl Mater Interfaces. 2020;12:38962–38975. doi: 10.1021/acsami.0c10599. [DOI] [PubMed] [Google Scholar]
- 18.González-Mayorga A, López-Dolado E, Gutiérrez MC, Collazos-Castro JE, Ferrer ML, Del Monte F, Serrano MC. Favorable biological responses of neural cells and tissue interacting with graphene oxide microfibers. ACS Omega. 2017;2:8253–8263. doi: 10.1021/acsomega.7b01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groves MN, Chan ASW, Malardier-Jugroot C, Jugroot M. Improving platinum catalyst binding energy to graphene through nitrogen doping. Chem Phys Lett. 2009;481:214–219. [Google Scholar]
- 20.Guo R, Li J, Chen C, Xiao M, Liao M, Hu Y, Liu Y, Li D, Zou J, Sun D, Torre V, Zhang Q, Chai R, Tang M. Biomimetic 3D bacterial cellulose-graphene foam hybrid scaffold regulates neural stem cell proliferation and differentiation. Colloids Surf B Biointerfaces. 2021;200:111590. doi: 10.1016/j.colsurfb.2021.111590. [DOI] [PubMed] [Google Scholar]
- 21.Guo R, Zhang S, Xiao M, Qian F, He Z, Li D, Zhang X, Li H, Yang X, Wang M, Chai R, Tang M. Accelerating bioelectric functional development of neural stem cells by graphene coupling:implications for neural interfacing with conductive materials. Biomaterials. 2016a;106:193–204. doi: 10.1016/j.biomaterials.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Qiu J, Liu J, Liu H. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep. 2017;7:5678. doi: 10.1038/s41598-017-06051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo W, Zhang X, Yu X, Wang S, Qiu J, Tang W, Li L, Liu H, Wang ZL. Self-powered electrical stimulation for enhancing neural differentiation of mesenchymal stem cells on graphene-poly(3,4-ethylenedioxythiophene) hybrid microfibers. ACS Nano. 2016b;10:5086–5095. doi: 10.1021/acsnano.6b00200. [DOI] [PubMed] [Google Scholar]
- 24.Hamid R, Averbeck MA, Chiang H, Garcia A, Al Mousa RT, Oh SJ, Patel A, Plata M, Del Popolo G. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J Urol. 2018;36:1517–1527. doi: 10.1007/s00345-018-2301-z. [DOI] [PubMed] [Google Scholar]
- 25.Hart CG, Karimi-Abdolrezaee S. Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J Neurosci Res. 2021 doi: 10.1002/jnr.24922. doi:10.1002/jnr.24922. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Heo C, Yoo J, Lee S, Jo A, Jung S, Yoo H, Lee YH, Suh M. The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes. Biomaterials. 2011;32:19–27. doi: 10.1016/j.biomaterials.2010.08.095. [DOI] [PubMed] [Google Scholar]
- 28.Hong SW, Lee JH, Kang SH, Hwang EY, Hwang YS, Lee MH, Han DW, Park JC. Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. Biomed Res Int. 2014;2014:212149. doi: 10.1155/2014/212149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignat SR, Lazăr AD, Şelaru A, Samoilă I, Vlăsceanu GM, Ioniţă M, Radu E, Dinescu S, Costache M. Versatile biomaterial platform enriched with graphene oxide and carbon nanotubes for multiple tissue engineering applications. Int J Mol Sci. 2019;20:3868. doi: 10.3390/ijms20163868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imani R, Emami SH, Faghihi S. Synthesis and characterization of an octaarginine functionalized graphene oxide nano-carrier for gene delivery applications. Phys Chem Chem Phys. 2015;17:6328–6339. doi: 10.1039/c4cp04301d. [DOI] [PubMed] [Google Scholar]
- 31.Iyer NR, Wilems TS, Sakiyama-Elbert SE. Stem cells for spinal cord injury:strategies to inform differentiation and transplantation. Biotechnol Bioeng. 2017;114:245–259. doi: 10.1002/bit.26074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jastrzębska AM, Kurtycz P, Olszyna AR. Recent advances in graphene family materials toxicity investigations. J Nanopart Res. 2012;14:1320. doi: 10.1007/s11051-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing X, Mi HY, Salick MR, Cordie TM, Peng XF, Turng LS. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mater Sci Eng C Mater Biol Appl. 2015;49:40–50. doi: 10.1016/j.msec.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 34.Johnson AP, Gangadharappa HV, Pramod K. Graphene nanoribbons:a promising nanomaterial for biomedical applications. J Control Release. 2020;325:141–162. doi: 10.1016/j.jconrel.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 35.Kalsi P, Thom M, Choi D. Histological effects of fibrin glue and synthetic tissue glues on the spinal cord:are they safe to use? Br J Neurosurg. 2017;31:695–700. doi: 10.1080/02688697.2017.1359491. [DOI] [PubMed] [Google Scholar]
- 36.Katoh H, Yokota K, Fehlings MG. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;13:248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Lee JH, Son SK, Kim KT. Biocompatible flexible carbon fabric for joule heaters with and without graphene oxide coating. J Nanosci Nanotechnol. 2021;21:3697–3700. doi: 10.1166/jnn.2021.19214. [DOI] [PubMed] [Google Scholar]
- 38.Kimura-Kuroda J, Teng X, Komuta Y, Yoshioka N, Sango K, Kawamura K, Raisman G, Kawano H. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci. 2010;43:177–187. doi: 10.1016/j.mcn.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolarcik CL, Catt K, Rost E, Albrecht IN, Bourbeau D, Du Z, Kozai TD, Luo X, Weber DJ, Cui XT. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J Neural Eng. 2015;12:016008. doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong FL, Wang XP, Li YN, Wang HX. The role of exosomes derived from cerebrospinal fluid of spinal cord injury in neuron proliferation in vitro. Artif Cells Nanomed Biotechnol. 2018;46:200–205. doi: 10.1080/21691401.2017.1304408. [DOI] [PubMed] [Google Scholar]
- 42.Kumamaru H, Lu P, Rosenzweig ES, Kadoya K, Tuszynski MH. Regenerating corticospinal axons innervate phenotypically appropriate neurons within neural stem cell grafts. Cell Rep. 2019;26:2329–2339.e4. doi: 10.1016/j.celrep.2019.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo WS, Yeh TS, Chang CY, Liu JC, Chen CH, So EC, Wu PC. Amino-functionalized nitrogen-doped graphene quantum dots for efficient enhancement of two-photon-excitation photodynamic therapy:functionalized nitrogen as a bactericidal and contrast agent. Int J Nanomedicine. 2020;15:6961–6973. doi: 10.2147/IJN.S242892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurantowicz N, Sawosz E, Jaworski S, Kutwin M, Strojny B, Wierzbicki M, Szeliga J, Hotowy A, Lipińska L, Koziński R, Jagiełło J, Chwalibog A. Interaction of graphene family materials with Listeria monocytogenes and Salmonella enterica. Nanoscale Res Lett. 2015;10:23. doi: 10.1186/s11671-015-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurapati R, Bonachera F, Russier J, Sureshbabu AR, Ménard-Moyon C, Kostarelos K, Bianco A. Covalent chemical functionalization enhances the biodegradation of graphene oxide. 2D Mater. 2018;5:015020. [Google Scholar]
- 46.Kurapati R, Russier J, Squillaci MA, Treossi E, Ménard-Moyon C, Del Rio-Castillo AE, Vazquez E, Samorì P, Palermo V, Bianco A. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small. 2015;11:3985–3994. doi: 10.1002/smll.201500038. [DOI] [PubMed] [Google Scholar]
- 47.Lee-Kubli CA, Lu P. Induced pluripotent stem cell-derived neural stem cell therapies for spinal cord injury. Neural Regen Res. 2015;10:10–16. doi: 10.4103/1673-5374.150638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B, Zhang XY, Yang JZ, Zhang YJ, Li WX, Fan CH, Huang Q. Influence of polyethylene glycol coating on biodistribution and toxicity of nanoscale graphene oxide in mice after intravenous injection. Int J Nanomedicine. 2014;9:4697–4707. doi: 10.2147/IJN.S66591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, Liu L, Jin G, Tang M, Cheng G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32:9374–9382. doi: 10.1016/j.biomaterials.2011.08.065. [DOI] [PubMed] [Google Scholar]
- 50.Li XP, Gan CL, Han ZY, Yan H, Chen DL, Li W, Li H, Fan XQ, Li DS, Zhu MH. High dispersivity and excellent tribological performance of titanate coupling agent modified graphene oxide in hydraulic oil. Carbon. 2020;165:238–250. [Google Scholar]
- 51.Li Y, Huang Z, Pu X, Chen X, Yin G, Wang Y, Miao D, Fan J, Mu J. Polydopamine/carboxylic graphene oxide-composited polypyrrole films for promoting adhesion and alignment of Schwann cells. Colloids Surf B Biointerfaces. 2020;191:110972. doi: 10.1016/j.colsurfb.2020.110972. [DOI] [PubMed] [Google Scholar]
- 52.Lima-Sousa R, de Melo-Diogo D, Alves CG, Cabral CSD, Miguel SP, Mendonça AG, Correia IJ. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater Sci Eng C Mater Biol. 2020;117:111294. doi: 10.1016/j.msec.2020.111294. [DOI] [PubMed] [Google Scholar]
- 53.Liu G, Shen H, Mao J, Zhang L, Jiang Z, Sun T, Lan Q, Zhang Z. Transferrin modified graphene oxide for glioma-targeted drug delivery:in vitro and in vivo evaluations. ACS Appl Mater Interfaces. 2013;5:6909–6914. doi: 10.1021/am402128s. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Wang S, Wang H, Lv C, Miao Y, Chen L, Yang S. Gold nanoparticles modified graphene foam with superhydrophobicity and superoleophilicity for oil-water separation. Sci Total Environ. 2021;758:143660. doi: 10.1016/j.scitotenv.2020.143660. [DOI] [PubMed] [Google Scholar]
- 55.López-Dolado E, González-Mayorga A, Gutiérrez MC, Serrano MC. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials. 2016;99:72–81. doi: 10.1016/j.biomaterials.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 56.López-Dolado E, González-Mayorga A, Portolés MT, Feito MJ, Ferrer ML, Del Monte F, Gutiérrez MC, Serrano MC. Subacute tissue response to 3d graphene oxide scaffolds implanted in the injured rat spinal cord. Adv Healthc Mater. 2015;4:1861–1868. doi: 10.1002/adhm.201500333. [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Do I, Drzal LT, Worden RM, Lee I. Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano. 2008;2:1825–1832. doi: 10.1021/nn800244k. [DOI] [PubMed] [Google Scholar]
- 58.Lukowiak A, Kedziora A, Strek W. Antimicrobial graphene family materials:progress, advances, hopes and fears. Adv Colloid Interface Sci. 2016;236:101–112. doi: 10.1016/j.cis.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Luo Z, Wang X, Yang D, Zhang S, Zhao T, Qin L, Yu ZZ. Photothermal hierarchical carbon nanotube/reduced graphene oxide microspherical aerogels with radially orientated microchannels for efficient cleanup of crude oil spills. J Colloid Interface Sci. 2020;570:61–71. doi: 10.1016/j.jcis.2020.02.097. [DOI] [PubMed] [Google Scholar]
- 60.Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, Zuo YY, Xia T, Liu S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. 2015;9:10498–10515. doi: 10.1021/acsnano.5b04751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malmsten M. Inorganic nanomaterials as delivery systems for proteins, peptides, DNA, and siRNA. Curr Opin Colloid Interface Sci. 2013;18:468–480. [Google Scholar]
- 62.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA American Society of Interventional Pain Physicians. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–70. [PubMed] [Google Scholar]
- 63.Marie Kalbacova, Antonin Broz, Jing Kong, Martin Kalbac. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon. 2010;48:4323–4329. [Google Scholar]
- 64.Mendes RG, Koch B, Bachmatiuk A, Ma X, Sanchez S, Damm C, Schmidt OG, Gemming T, Eckert J, Rümmeli MH. A size dependent evaluation of the cytotoxicity and uptake of nanographene oxide. J Mater Chem B. 2015;3:2522–2529. doi: 10.1039/c5tb00180c. [DOI] [PubMed] [Google Scholar]
- 65.Min KJ, Kim TH, Choi JW. Magnetic force-driven graphene patterns to direct synaptogenesis of human neuronal cells. Materials (Basel) 2017;10:1151. doi: 10.3390/ma10101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mortazavi Y, Sheikhsaran F, Khamisipour GK, Soleimani M, Teimuri A, Shokri S. The evaluation of nerve growth factor over expression on neural lineage specific genes in human mesenchymal stem cells. Cell J. 2016;18:189–196. doi: 10.22074/cellj.2016.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narayanan KB, Kim HD, Han SS. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surf B Biointerfaces. 2020;185:110579. doi: 10.1016/j.colsurfb.2019.110579. [DOI] [PubMed] [Google Scholar]
- 68.Ou L, Song B, Liang H, Liu J, Feng X, Deng B, Sun T, Shao L. Toxicity of graphene-family nanoparticles:a general review of the origins and mechanisms. Part Fibre Toxicol. 2016;13:57. doi: 10.1186/s12989-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palejwala AH, Fridley JS, Mata JA, Samuel EL, Luerssen TG, Perlaky L, Kent TA, Tour JM, Jea A. Biocompatibility of reduced graphene oxide nanoscaffolds following acute spinal cord injury in rats. Surg Neurol Int. 2016;7:75. doi: 10.4103/2152-7806.188905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan S, Qi Z, Li Q, Ma Y, Fu C, Zheng S, Kong W, Liu Q, Yang X. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif Cells Nanomed Biotechnol. 2019;47:651–664. doi: 10.1080/21691401.2019.1575843. [DOI] [PubMed] [Google Scholar]
- 71.Qu D, Zheng M, Li J, Xie Z, Sun Z. Tailoring color emissions from n-doped graphene quantum dots for bioimaging applications. Light Sci Appl. 2015;4:e364. [Google Scholar]
- 72.Raslan A, Saenz Del Burgo L, Ciriza J, Pedraz JL. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int J Pharm. 2020;580:119226. doi: 10.1016/j.ijpharm.2020.119226. [DOI] [PubMed] [Google Scholar]
- 73.Rauti R, Secomandi N, Martín C, Bosi S, Severino FPU, Scaini D, Prato M, Vázquez E, Ballerini L. Tuning neuronal circuit formation in 3d polymeric scaffolds by introducing graphene at the bio/material interface. Adv Biosyst. 2020;4:e1900233. doi: 10.1002/adbi.201900233. [DOI] [PubMed] [Google Scholar]
- 74.Robinson J, Lu P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp Neurol. 2017;291:87–97. doi: 10.1016/j.expneurol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez-Losada N, Romero P, Estivill-Torrús G, Guzmán de Villoria R, Aguirre JA. Cell survival and differentiation with nanocrystalline glass-like carbon using substantia nigra dopaminergic cells derived from transgenic mouse embryos. PLoS One. 2017;12:e0173978. doi: 10.1371/journal.pone.0173978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury:current concepts and treatment update. Arq Neuropsiquiatr. 2017;75:387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 77.Ryoo SR, Kim YK, Kim MH, Min DH. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes:proliferation, focal adhesion, and gene transfection studies. ACS Nano. 2010;4:6587–6598. doi: 10.1021/nn1018279. [DOI] [PubMed] [Google Scholar]
- 78.Sahni D, Jea A, Mata JA, Marcano DC, Sivaganesan A, Berlin JM, Tatsui CE, Sun Z, Luerssen TG, Meng S, Kent TA, Tour JM. Biocompatibility of pristine graphene for neuronal interface. J Neurosurg Pediatr. 2013;11:575–583. doi: 10.3171/2013.1.PEDS12374. [DOI] [PubMed] [Google Scholar]
- 79.Saravanan S, Vimalraj S, Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed Pharmacother. 2018;107:908–917. doi: 10.1016/j.biopha.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 80.Sasidharan A, Panchakarla LS, Sadanandan AR, Ashokan A, Chandran P, Girish CM, Menon D, Nair SV, Rao CN, Koyakutty M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small. 2012;8:1251–1263. doi: 10.1002/smll.201102393. [DOI] [PubMed] [Google Scholar]
- 81.Schinwald A, Murphy F, Askounis A, Koutsos V, Sefiane K, Donaldson K, Campbell CJ. Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology. 2014;8:824–832. doi: 10.3109/17435390.2013.831502. [DOI] [PubMed] [Google Scholar]
- 82.Seabra AB, Paula AJ, de Lima R, Alves OL, Durán N. Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol. 2014;27:159–168. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 83.Serrano MC, Feito MJ, González-Mayorga A, Diez-Orejas R, Matesanz MC, Portolés MT. Response of macrophages and neural cells in contact with reduced graphene oxide microfibers. Biomater Sci. 2018;6:2987–2997. doi: 10.1039/c8bm00902c. [DOI] [PubMed] [Google Scholar]
- 84.Shi J, Guo J, Bai G, Chan C, Liu X, Ye W, Hao J, Chen S, Yang M. A graphene oxide based fluorescence resonance energy transfer (FRET) biosensor for ultrasensitive detection of botulinum neurotoxin A (BoNT/A) enzymatic activity. Biosens Bioelectron. 2015;65:238–244. doi: 10.1016/j.bios.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 85.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure:role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7:a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slusarczyk W, Olakowska E, Larysz-Brysz M, Woszczycka-Korczyńska I, de Carrillo DG, Węglarz WP, Lewin-Kowalik J, Marcol W. Use of ebselen as a neuroprotective agent in rat spinal cord subjected to traumatic injury. Neural Regen Res. 2019;14:1255–1261. doi: 10.4103/1673-5374.251334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sofo JO, Chaudhari AS, Barber GD. Graphane:a two-dimensional hydrocarbon. Phys Rev B. 2006;75:153401. [Google Scholar]
- 89.Solanki A, Chueng ST, Yin PT, Kappera R, Chhowalla M, Lee KB. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv Mater. 2013;25:5477–5482. doi: 10.1002/adma.201302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song Q, Jiang Z, Li N, Liu P, Liu L, Tang M, Cheng G. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials. 2014;35:6930–6940. doi: 10.1016/j.biomaterials.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Su C, Loh KP. Carbocatalysts:graphene oxide and its derivatives. Acc Chem Res. 2013;46:2275–2285. doi: 10.1021/ar300118v. [DOI] [PubMed] [Google Scholar]
- 92.Tabish TA, Pranjol MZI, Hayat H, Rahat AAM, Abdullah TM, Whatmore JL, Zhang S. In vitro toxic effects of reduced graphene oxide nanosheets on lung cancer cells. Nanotechnology. 2017;28:504001. doi: 10.1088/1361-6528/aa95a8. [DOI] [PubMed] [Google Scholar]
- 93.Tian Z, Sun L, Tian H, Cao K, Bai S, Li J, Zhu Q. 3D graphene oxide hydrogel derived from waste toner as adsorbent. J Nanosci Nanotechnol. 2021;21:5275–5281. doi: 10.1166/jnn.2021.19339. [DOI] [PubMed] [Google Scholar]
- 94.Tu Q, Pang L, Wang L, Zhang Y, Zhang R, Wang J. Biomimetic choline-like graphene oxide composites for neurite sprouting and outgrowth. ACS Appl Mater Interfaces. 2013;5:13188–13197. doi: 10.1021/am4042004. [DOI] [PubMed] [Google Scholar]
- 95.Usmani S, Franceschi Biagioni A, Medelin M, Scaini D, Casani R, Aurand ER, Padro D, Egimendia A, Ramos Cabrer P, Scarselli M, De Crescenzi M, Prato M, Ballerini L. Functional rewiring across spinal injuries via biomimetic nanofiber scaffolds. Proc Natl Acad Sci U S A. 2020;117:25212–25218. doi: 10.1073/pnas.2005708117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valencia AM, Valencia CH, Zuluaga F, Grande-Tovar CD. Synthesis and fabrication of films including graphene oxide functionalized with chitosan for regenerative medicine applications. Heliyon. 2021;7:e07058. doi: 10.1016/j.heliyon.2021.e07058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernández C, Tapiador N, Sevilla M, Morejón C, Montilla J, Martínez F, Marín E, Bustamante S, Vázquez D, Carballido J, Rodríguez A, Martínez P, García C, Ovejero M, Fernández MV, Neurological Cell Therapy Group Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19:349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current options for cell therapy in spinal cord injury. Trends Mol Med. 2017;23:831–849. doi: 10.1016/j.molmed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Wang H, Gu W, Xiao N, Ye L, Xu Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int J Nanomedicine. 2014;9:1433–1442. doi: 10.2147/IJN.S58783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J, Cheng Y, Chen L, Zhu T, Ye K, Jia C, Wang H, Zhu M, Fan C, Mo X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019a;84:98–113. doi: 10.1016/j.actbio.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 101.Wang J, Zou W, Ma J, Liu J. Biomaterials and gene manipulation in stem cell-based therapies for spinal cord injury. Stem Cells Dev. 2019b;28:239–257. doi: 10.1089/scd.2018.0169. [DOI] [PubMed] [Google Scholar]
- 102.Wang L, Liu X, Fu J, Ning X, Zhang M, Jiang Z, Cheng G, Zhu Y, Zhang Z. Release of methylene blue from graphene oxide-coated electrospun nanofibrous scaffolds to modulate functions of neural progenitor cells. Acta Biomater. 2019c;88:346–356. doi: 10.1016/j.actbio.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 103.White RL, White CM, Turgut H, Massoud A, Tian ZR. Comparative studies on copper adsorption by graphene oxide and functionalized graphene oxide nanoparticles. J Taiwan Inst Chem Eng. 2018;85:18–28. [Google Scholar]
- 104.Windle WF. Regeneration of axons in the vertebrate central nervous system. Physiol Rev. 1956;36:427–440. doi: 10.1152/physrev.1956.36.4.427. [DOI] [PubMed] [Google Scholar]
- 105.Yang B, Wang PB, Mu N, Ma K, Wang S, Yang CY, Huang ZB, Lai Y, Feng H, Yin GF, Chen TN, Hu CS. Graphene oxide-composited chitosan scaffold contributes to functional recovery of injured spinal cord in rats. Neural Regen Res. 2021;16:1829–1835. doi: 10.4103/1673-5374.306095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang D, Li T, Xu M, Gao F, Yang J, Yang Z, Le W. Graphene oxide promotes the differentiation of mouse embryonic stem cells to dopamine neurons. Nanomedicine (Lond) 2014;9:2445–2455. doi: 10.2217/nnm.13.197. [DOI] [PubMed] [Google Scholar]
- 107.Yang K, Lee J, Lee JS, Kim D, Chang GE, Seo J, Cheong E, Lee T, Cho SW. Graphene oxide hierarchical patterns for the derivation of electrophysiologically functional neuron-like cells from human neural stem cells. ACS Appl Mater Interfaces. 2016;8:17763–17774. doi: 10.1021/acsami.6b01804. [DOI] [PubMed] [Google Scholar]
- 108.Yang K, Wan J, Zhang S, Tian B, Zhang Y, Liu Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials. 2012;33:2206–2214. doi: 10.1016/j.biomaterials.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 109.Yang XY, Zhang XY, Liu ZF, Ma YF, Huang Y, Chen YS. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C. 2018;112:17554–17558. [Google Scholar]
- 110.Yao L, Shanley L, McCaig C, Zhao M. Small applied electric fields guide migration of hippocampal neurons. J Cell Physiol. 2008;216:527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- 111.Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, Ma D, Ma G, Su Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012;33:4013–4021. doi: 10.1016/j.biomaterials.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 112.Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J. Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int J Biol Sci. 2021;17:1339–1352. doi: 10.7150/ijbs.54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J, Zhang F, Yang H, Huang X, Liu H, Zhang J, Guo S. Graphene oxide as a matrix for enzyme immobilization. Langmuir. 2010a;26:6083–6085. doi: 10.1021/la904014z. [DOI] [PubMed] [Google Scholar]
- 114.Zhang K, Zheng H, Liang S, Gao C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016;37:131–142. doi: 10.1016/j.actbio.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Zhang L, He Y, Zhu L, Yang C, Niu QH, An CL. In-situ alkylated graphene as oil dispersible additive for friction and wear reduction. Indust Eng Chem Res ACS. 2017a doi:10.1021/acs.iecr.7b01338. [Google Scholar]
- 116.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010b;6:537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Q, Du QY, Zhao YN, Chen FX, Wang ZJ, Zhang YX, Ni H, Deng HB, Li YP, Chen Y. Graphene oxide-modified electrospun polyvinyl alcohol nanofibrous scaffolds with potential as skin wound dressings. Rsc Adv. 2017b;7:28826–28836. [Google Scholar]
- 118.Zhang Q, Liu X, Meng H, Liu S, Zhang C. Reduction pathway-dependent cytotoxicity of reduced graphene oxide. Environ Sci Nano. 2018;5:1361–1371. [Google Scholar]
- 119.Zhang Y, Ali SF, Dervishi E, Xu Y, Li Z, Casciano D, Biris AS. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano. 2010c;4:3181–3186. doi: 10.1021/nn1007176. [DOI] [PubMed] [Google Scholar]
- 120.Zhou K, Yu P, Shi X, Ling T, Zeng W, Chen A, Yang W, Zhou Z. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano. 2019;13:9595–9606. doi: 10.1021/acsnano.9b04723. [DOI] [PubMed] [Google Scholar]
- 121.Zhou T, Zhou X, Xing D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials. 2014;35:4185–4194. doi: 10.1016/j.biomaterials.2014.01.044. [DOI] [PubMed] [Google Scholar]



