Fig. 4. CRISPR screen using MIC-Drop identifies novel genes responsible for cardiovascular development.
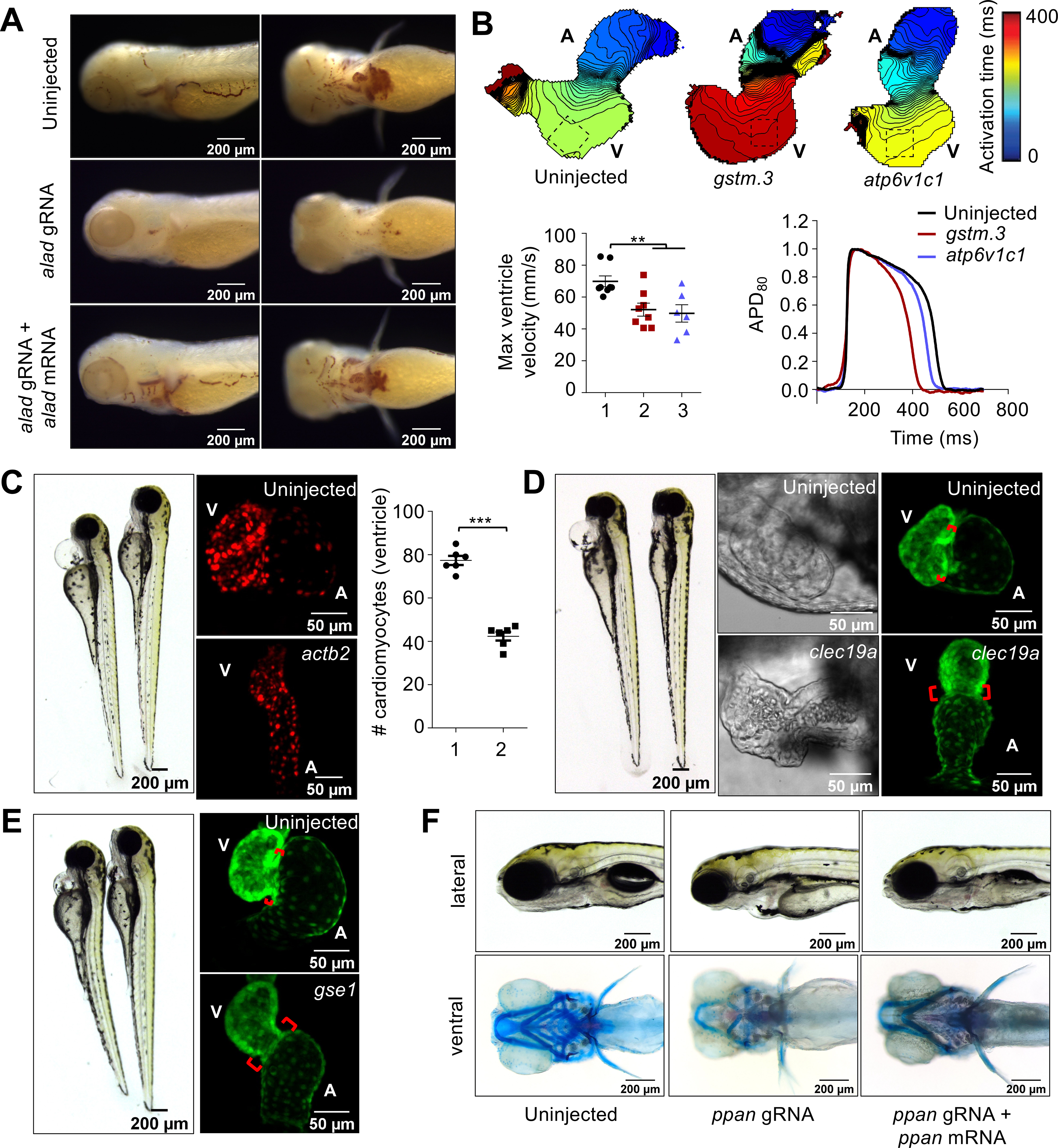
(A) o-dianisidine staining shows loss of alad results in porphyria, which can be rescued by co-injection of alad mRNA. (B) Loss of gstm.3 or atp6v1c1 results in abnormal cardiac electrophysiology. Isochronal maps and action potential measurements reveal reduced conduction velocities, and shorter ventricular action potential duration in the gstm.3 and atp6v1c1 crispants relative to uninjected controls 1: Uninjected; 2: gstm.3 crispants; 3. atp6v1c1 crispants. Data are presented as mean ± sem (** = p≤0.01; *** = p≤0.001). Loss of (C) actb2 (D) clec19a (E) gse1, and (F) ppan result in distinct cardiac malformations. actb2 crispants have a small ventricle with reduced number of ventricular cardiomyocytes 1: Control; 2: actb2-targeting gRNAs. (C). Loss of clec19a and gse1 result in abnormal morphogenesis and an extended atrioventricular canal relative to wildtype embryos (D-E). Alcian blue staining of ppan crispants shows abnormal jaw and skull development, which is rescued by ppan mRNA injection. The embryos also display cardiac edema, and a silent ventricle (F).
