Optimisation of the reaction conditionsa.
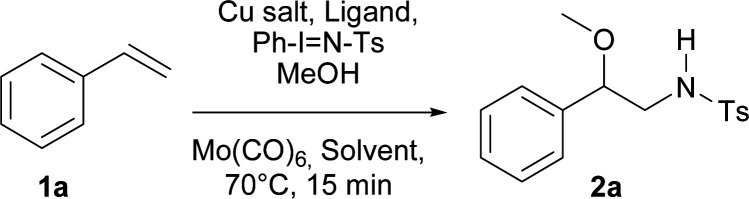
| ||||
|---|---|---|---|---|
| Entry | Copper salt 10% | Ligand 10% | Solvent | Yieldb,c[%] |
| 1 | Cu(MeCN)4BF4 | Neocuproine | DCE | (48) |
| 2 | — | Neocuproine | DCE | — |
| 3 | Cu(BF4)2·H2O | Neocuproine | DCE | 53(54) |
| 4 | Cu(BF 4 ) 2 ·H 2 O | Neocuproine | MeNO 2 | 74(75) |
| 5 | Cu(BF4)2·H2O | Neocuproine | CH3CN | (45) |
| 6 | Cu(BF4)2·H2O | Neocuproine | Toluene | (53) |
| 7 | Cu(BF4)2·H2O | Neocuproine | Ph-Cl | (66) |
| 8 | Cu(BF4)2·H2O | Neocuproine | EtOAc | (27) |
| 9 | Cu(BF4)2·H2O | Neocuproine | MeOH | (66) |
| 10 | Cu(BF4)2·H2O | 2,2′-Bipyridine | MeNO2 | (57) |
| 11 | Cu(BF4)2·H2O | 1,10-Phenanthroline | MeNO2 | (39) |
| 12d | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (36) |
| 13e | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (72) |
| 15f | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (73) |
| 16g | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (69) |
| 17h | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (57) |
| 18i | Cu(BF4)2·H2O | Neocuproine | MeNO2 | (36) |
Reactions were carried out with 1a (1.0 mmol) in 3.0 mL of solvent, Ph-I N-Ts (1.5 eq.), MeOH (10 eq.), Mo(CO)6 (0.25 eq) in an open tube, unless otherwise noted.
NMR-determined yields in a 0.20 mmol scale reaction of 1a using 1,3,5-trimethoxybenzene as internal standard are shown in parentheses.
Isolated yields.
Catalyst 5%.
30 min.
MeOH (5.0 eq.).
MeOH (2.0 eq).
Mo(CO)6 (0.1 eq.).
Neocuproine (20%).
