Abstract
Butenafine (N-4-tert-butylbenzyl-N-methyl-1-naphtalenemethylamine hydrochloride) is an antifungal agent of the benzylamine class that has excellent therapeutic efficacy and a remarkably long duration of action when applied topically to treat various mycoses. Given the lipophilic nature of the molecule, efficacy may be related to an interaction with cell membrane phospholipids and permeabilization of the fungal cell wall. Similarly, high lipophilicity could account for the long duration of action, since fixation to lipids in cutaneous tissues might allow them to act as local depots for slow release of the drug. We have therefore used computer-assisted conformational analysis to investigate the interaction of butenafine with lipids and extended these observations with experimental studies in vitro using liposomes. Conformational analysis of mixed monolayers of phospholipids with the neutral and protonated forms of butenafine highlighted a possible interaction with both the hydrophilic and hydrophobic domains of membrane phospholipids. Studies using liposomes demonstrated that butenafine increases membrane fluidity [assessed by fluorescence polarization of 1-(4-trimethylammonium-phenyl)-6-phenyl-1,3,5-hexatriene and 1,6-diphenylhexatriene] and membrane permeability (studied by release of calcein from liposomes). The results show, therefore, that butenafine readily interacts with lipids and is incorporated into membrane phospholipids. These findings may help explain the excellent antifungal efficacy and long duration of action of this drug when it is used as a topical antifungal agent in humans.
Butenafine (N-4-tert-butylbenzyl-N-methyl-1-naphtalenemethylamine hydrochloride), a broad-spectrum topical antifungal agent (21), shows excellent therapeutic efficacy in humans with dermatomycoses (13, 22, 34, 36, 44, 50, 54). In vitro, the MIC and the minimal fungicidal concentration of butenafine against Trichophyton mentagrophytes and Microsporum canis were 0.012 to 0.05 mg/liter, i.e., 4 to 130 times lower than those for naftifine, tolnaftate, clotrimazole, and bifonazole (13, 36), other well-known antifungal drugs. Of additional interest is the fact that, in contrast to imidazole and triazole antifungals, butenafine does not interact with cytochrome P450-dependent enzymes (13) and is, therefore, unlikely to cause toxicity via untoward drug-drug interactions.
The efficacy of butenafine might be attributed to its ability to inhibit sterol synthesis by blocking squalene epoxidation (24, 25). This would lead to depletion of ergosterol, an essential lipid component of the fungal membrane; accumulation of squalene; and alteration in membrane function. At high concentrations, the damaging effect of butenafine on the cell membrane might play a major role in its anticandidal activity (27).
One of the major characteristics of butenafine is its ability to provide long-lasting antifungal activity. Topical application of butenafine produces residual fungicidal concentrations in the skin (and particularly in the stratum corneum) that remain for at least 72 h (2, 3). In short-term clinical trials (<4 weeks), antifungal efficacy was maintained up to 5 weeks after the end of the treatment (27, 36). This remarkably long effect is probably due to the lipophilic nature of butenafine (characterized by a high partition coefficient [≈30 in an octanol-water system]).
Although such data have been known for some time, nothing has been published on the molecular interactions between butenafine and lipids. The present study investigates this point further, using experimental and conformational studies. Here, we report the effect of butenafine on the permeability of lipid vesicles (liposomes) and the effect on membrane fluidity. The results are discussed together with those obtained by conformational analysis of the interaction between butenafine and phospholipids.
MATERIALS AND METHODS
Experimental studies. (i) Preparation of liposomes.
Small unilamellar vesicles were prepared from a sterol (cholesterol), glycerophospholipids (phosphatidylcholine and phosphatidylinositol), and a phosphosphingolipid (sphingomyelin) (molar ratio, 5.5:4:3:4 [33.3, 24.2, 18.3, and 24.2%, respectively]). The vesicles were prepared by sonication in Tris buffer (pH 8.0) (10 mM Tris, 150 mM NaCl, 0.1 mM EDTA, 1 mM NaN3) as described earlier (30). In brief, dry lipid films were obtained by evaporation of the solvent of lipids (CHCl3-CH3OH; 2:1) in a rotavapor with overnight dessiccation. The lipid film was then resuspended in buffer or in calcein solution (see “Permeability studies”) and incubated for 1 h at 37°C in a nitrogen atmosphere. The suspension was sonicated at 4°C under a stream of nitrogen using a Labsonic-L sonotrode (Braun Biotech International, Melsungen, Germany) set at 50 W for five 2-min periods with a 1-min cooling interval until the opaque suspension became translucent. The preparations were then centrifuged at 1,000 × g for 10 min (CRU-5000; Damon IEC) to remove particulate matter. The actual phospholipid concentration of each preparation was determined by phosphorus assay (4). The total lipid concentration was calculated assuming similar recovery of phospholipids and cholesterol. The average diameter of liposomes evaluated by quasielastic light spectroscopy using a Nano-Sizer N4MD particle analyzer (Coulter Electronics Ltd., Luton, England) was typically 100 ± 20 nm. The liposomes were stored under nitrogen and used within 24 h.
(ii) Permeability studies.
Leakage of entrapped, self-quenched calcein from liposomes was monitored by the increase of fluorescence subsequent to dilution (56). The dry lipid films were hydrated to a final concentration of 2 mg of lipid/ml in a solution of purified calcein (8.9 mM). The final solution had an osmolarity of 353 mosmol/kg (measured by the freezing point technique [Advanced Cryomatic osmometer, model 3C2; Advanced Instruments Inc., Needham Heights, Mass.]). After the preparation of vesicles, the unencapsulated dye was discarded by the minicolumn centrifugation technique of Lelkes (31). The recovery of liposomes was determined by measuring their phospholipid content, using the phosphorus assay (4), and was typically >90%. The liposomes were diluted to a final lipid concentration of 5 μM (using the average molecular weight of the constituent lipids) in Tris buffer (10 mM Tris, 166 mM NaCl, 0.1 mM EDTA, 1 mM NaN3) (pH 8; 353 mosm/kg). Increasing concentrations of butenafine were added to the liposomes. The mixture was vortexed for 20 s, and the first fluorescence determination was made 1 min after addition of the drug. All fluorescence determinations were performed at room temperature on an LS 30 fluorescence spectrophotometer (Perkin-Elmer Ltd., Beaconsfield, United Kingdom) using excitation and emission wavelengths of 472 and 516 nm, respectively. The percentage of calcein released under the influence of butenafine was defined as [(Ft − Fcontr)/(Ftot − Fcontr)] × 100, where Ft is the fluorescence signal measured at time t in the presence of the drug, Fcontr is the fluorescence signal measured at the same time in the control liposomes, and Ftot is the total fluorescence signal obtained after complete disruption of liposomes by ultrasound (verified by quasielastic light spectroscopy), which caused complete release of calcein.
(iii) Fluorescence polarization studies.
Membrane fluidity was studied by measuring the degree of fluorescence polarization of 1,6-diphenylhexatriene (DPH), and 1-(4-trimethylammonium-phenyl)-6-phenyl-1,3,5-hexatriene (TMA-DPH) dyes as a function of temperature according to the method of Shinitzky and Barenholz (51). Liposomes, prepared in Tris buffer (10 mM Tris, 150 mM NaCl, 0.1 mM EDTA, 1 mM NaN3, pH 8) at a final lipid concentration of 300 μM, were preincubated with DPH (1 mol/209 mol of lipids) or TMA-DPH (1mol/277 mol of lipids) for 3 h at 37°C. After incubation with butenafine (0.5 h at 37°C), samples were brought to 60°C for 15 min, and the temperature was gradually decreased to 5°C at a constant rate of 0.83°C/min using a programmable bath (Haake, Karlsruhe, Germany). The samples were gently stirred throughout, and the temperature was continuously monitored. Fluorescence polarization was measured with a Perkin-Elmer LS-50 fluorimeter equipped for polarization measurements and operating at excitation and emission wavelengths of 365 nm (slit-width, 5 nm) and 427 nm (slit-width, 4 nm), respectively. The degree of polarization is expressed as [(Ipar − Iper)/(Ipar + Iper)], where Ipar and Iper are the intensities of the light emitted in the planes parallel and perpendicular to that of the polarized excitation light, respectively.
Conformational analysis.
Models of the neutral and charged molecular structures of butenafine were built using Hyperchem 5.0 software (Autodesk, Sausalito, Calif.). The method used for the theoretical conformational analysis of butenafine is based on a semiempirical method described elsewhere (8). The total conformational energy, i.e., the sum of the contributions resulting from Van der Waals interactions, the torsional potential, and the electrostatic interactions, is calculated for a large number of conformations, using a systematic analysis of all torsional angles α (see Fig. 3). Conformations for the lowest internal energy of butenafine were processed using the Simplex energy minimization procedure (41). This procedure reduces the total internal energy due to rotation of torsional axes in a medium of low dielectric constant representative of the hydrophobic part of the membrane at the lipid-water interface. The energy-refined molecular models were then oriented at the lipid-water interface taking into account the positions of the hydrophobic and hydrophilic centers (8). As the next step, butenafine was surrounded with phospholipid molecules using the Hypermatrix procedure (10). This method is based on a strategy in which the molecular structure of butenafine is fixed in the position of its orientation at the air-water interface (11). The first phospholipid molecule is then positioned at this interface and allowed to move along the x axis in 1-Å steps. At each position, the phospholipid molecule is rotated by 30° steps around its long z′ axis and around the butenafine molecule. For each position, the energy of intermolecular interactions is calculated as the sum of the London-Van der Waals energy of interaction (EVdW), the electrostatic interaction (Ecb), and the transfer energy of atoms or groups of atoms from a hydrophobic to a hydrophilic phase (Etr). A second phospholipid molecule is then added and moved by 1-Å steps along the z′ axis perpendicular to the interface, which is rotated by 5° steps with respect to the z axis (the central molecule axis). This approach, in which the structure of the lowest interaction energy is finally retained, was limited to the number of phospholipid molecules required to surround a molecule of butenafine. Butenafine was assessed using three different phospholipids: di-palmitoyl-phosphatidylethanolamine (DPPE), di-palmitoyl-phosphatidylcholine (DPPC), and the two isomers of palmitoyl-oleoyl-phosphatidylcholine (POPC1 and POPC2). This method has proven useful for describing the interactions of several drugs with lipid membranes (e.g., aminoglycosides, macrolides, adriamycin, ethidium bromide, antimycotics, propranolol, various alcohols, ionophores, and peptides) (7, 15, 30, 38–40, 55). It should be noted that there is good agreement between results obtained using these methods and the results of neutron, X-ray diffraction, and polarized infrared spectroscopy studies of lipids (10, 14), ionophores (9, 17), and peptides (5, 12).
FIG. 3.
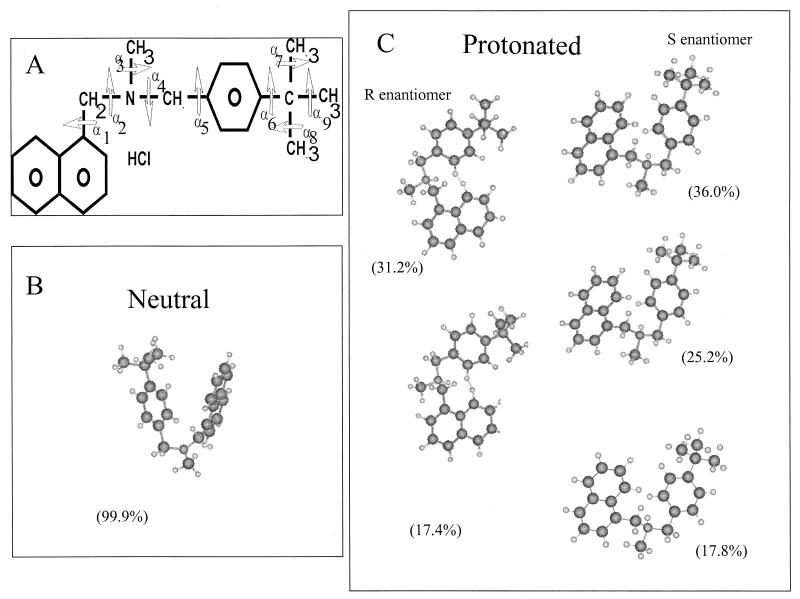
(A) Primary structure of butenafine; the torsion angles are annotated α1 to α9. (B and C) Selected structures of butenafine (probability, >15%) obtained by the Simplex energetic minimization procedure. The percentages of probability of the neutral form (B) and the protonated forms (R and S enantiomers) (C) are indicated for each structure.
All calculations were performed using the PC-Tammo (theoretical analysis of molecular membrane organization) (6) and PC-MSA (molecular structure analysis) (7) programs. Graphic visualizations were performed using the WinMGM software (49) from Ab Initio Technology (Obernai, France). Detailed information on computer programs and their characteristics is available from R. Brasseur.
Materials.
Butenafine, supplied by UCB (Brussels, Belgium), was dissolved in methanol. Egg phosphatidylcholine and phosphatidylinositol (grade 1) were purchased from Lipid Products (Redhill, United Kingdom), and sphingomyelin and cholesterol were purchased from Sigma Chemical Co. DPH and TMA-DPH were obtained from Molecular Probes Inc. (Eugene, Oreg.). Calcein, purchased from the Sigma Chemical Co, was purified by chromatography on Sephadex LH-20 (31), and the purity of the final product was checked by thin-layer chromatography on silica gel G using CH3OH-NH4OH 28% (9:1.5 [vol/vol]) as the mobile phase. Melittin came from Fluka (Buchs, Switzerland). Other reagents were obtained from E. Merck (Darmstadt, Germany) and were of analytical grade.
RESULTS
Experimental studies. (i) Calcein permeability.
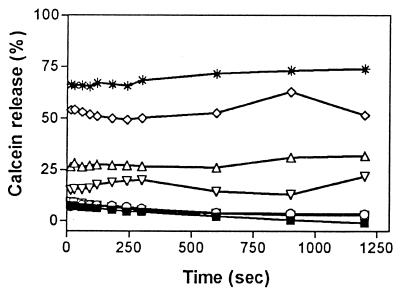
Calcein, a polar molecule with a molecular weight of 622.5, has been widely used to study the permeability of lipid bilayers (1). Figure 1 shows that butenafine promoted the release of calcein from liposomes at concentrations of ≥625 μM (200 μg/ml). The release was rapid (half-life, <60 s), and the extent of release was more marked when concentrations of butenafine increased over the range 625 to 2,500 μM (200 to 800 μg/ml). No additional release was observed at butenafine concentrations higher than 2,500 μM (800 μg/ml). The effect of butenafine on liposome permeability was, however, less marked than that of melittin, a well-known porogenic agent, which induced 70% calcein release at much lower concentrations (1 μM).
FIG. 1.
Time dependence of calcein release from liposomes (5 μM) made of cholesterol, phosphatidylcholine, phosphatidylinositol, and sphingomyelin (33.3, 24.2, 18.3, and 24.2%) upon incubation at 37°C in the presence of increasing concentrations of butenafine. The ordinate shows the amount of calcein released in the presence of the agent under study as a percentage of the total amount released by sonication. The concentrations of butenafine ranged from 25 to 2,500 μM (8 to 800 μg/ml). ■ , butenafine solvent (control); □, butenafine (25 μM; 8 μg/ml); ○, butenafine (250 μM; 80 μg/ml); ▿, butenafine (625 μM; 200 μg/ml); Δ, butenafine (1,250 μM; 400 μg/ml); ◊, butenafine (2,500 μM; 800 μg/ml); ∗, results obtained with melittin, used as a positive control (concentration, 1 μM). Each value is the mean of three independent experimental determinations. Standard deviations were <2% in each case.
(ii) Fluorescence polarization.
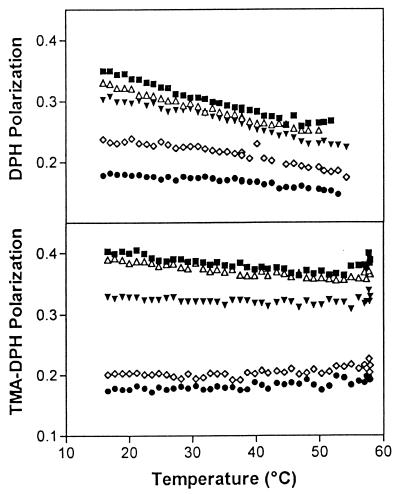
To investigate the influence of butenafine on membrane fluidity, we examined its effect on the fluorescence polarization of DPH (Fig. 2, top panel) and of the protonated derivative, TMA-DPH (Fig. 2, bottom panel). The results show that the degree of polarization of the two probes decreased linearly in control liposomes when the temperature of the sample was increased (40). The variation of polarization upon warming was, however, lower with TMA-DPH than with DPH, which indicates a reduced mobility of the alkyl chains closer to the interface compared to that of those lying deeper in the hydrophobic domain. Moreover, the polarization value recorded with TMA-DPH was higher than with DPH at each temperature, indicating an intrinsically higher rigidity of the membrane domain closer to the interface. At all temperatures investigated, the addition of butenafine at concentrations of ≥250 μM (80 μg/ml) significantly reduced, in a concentration-dependent fashion, the degree of polarization of both DPH and TMA-DPH, which indicates an increase in membrane fluidity.
FIG. 2.
Variation in polarization of DPH (top panel) and TMA-DPH (bottom panel) fluorescence incorporated in liposomes made of cholesterol, phosphatidylcholine, phosphatidylinositol, and sphingomyelin (33.3, 24.2, 18.3, and 24.2%). Vesicles (300 μM) were incubated with butenafine at increasing concentrations. ■, butenafine solvent (control); Δ, butenafine (25 μM; 8 μg/ml); ▾, butenafine (250 μM; 80 μg/ml); ◊, butenafine (500 μM; 160 μg/ml); ●, butenafine (1,000 μM; 320 μg/ml). Each point is the mean value of four independent experiments; standard deviations are not shown for the sake of clarity.
Conformational analysis.
Models for three forms of butenafine were constructed, a neutral form and two charged enantiomers (R and S), according to the two possible positions of the proton on the trigonal nitrogen atom. The molecular structure and the definition of the nine angles of torsion of butenafine (α 1 to α 9) are plotted in Fig. 3A. The angles α2, α5, α 6, α 8, and α 9 had undergone a systematic rotation by steps of 60°, and 7,776 (or 65) conformations were generated. The most likely conformation for each neutral or protonated form (selected using a Boltzmann statistic for each conformer) was obtained after this systematic analysis. Conformations with a probability of existence lower than 2% were rejected. For the neutral form and the R and S enantiomers, 4, 12, and 10 possible conformations were calculated, respectively. All retained conformers underwent energy minimization using the Simplex procedure (41), and statistical analysis of these showed that one, two, and three structures of the neutral form and the R and S enantiomers, respectively, had conformation probabilities over 15%. The neutral form and the S enantiomer had globular forms and were folded such that the aromatic rings (benzene and naphthalene) were overlaid to create a resonance effect between their electronic clouds. In contrast, the R enantiomer was flat and the aromatic groups were almost in the same plane (Fig. 3B and C).
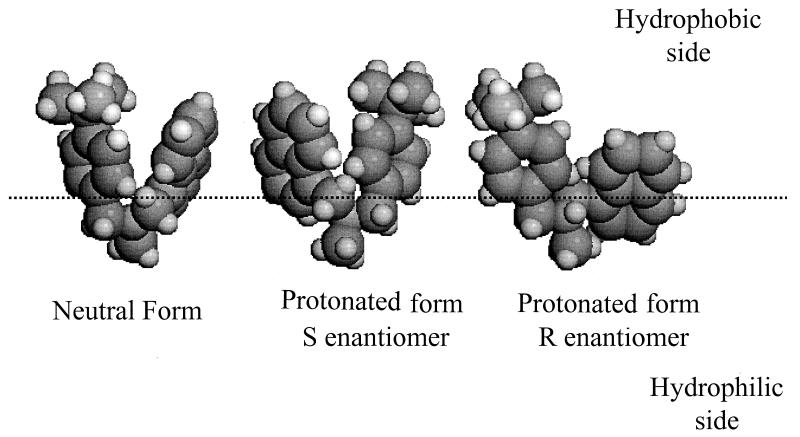
When the three major structures of each form were oriented in relation to the lipid-water interface (Fig. 4), their positions were very similar. They showed the obvious hydrophobic nature of the molecules, and the aromatic rings clustered in the hydrophobic phase with the nitrogen atom (protonated or not) at the interface. The proton carried by the nitrogen in the R and S enantiomers was exactly in the interface plane, and the methyl group on the nitrogen atom was always in the hydrophilic phase. The conformation similarity between the neutral and protonated (S enantiomer) forms was also observed after calculation of the butenafine area at the interface, 70 and 65 Å2, respectively. The flatter form of the R enantiomer logically produced a larger area: 91 Å2. Calculation of the molecular hydrophobic potentials also demonstrated the hydrophobic nature of butenafine, and the potentials of the three forms studied were entirely hydrophobic.
FIG. 4.
Orientations at the lipid-water interface of the three most likely forms of neutral butenafine and protonated butenafine represented in space-filling stereoviews. The interface is indicated by the dotted line.
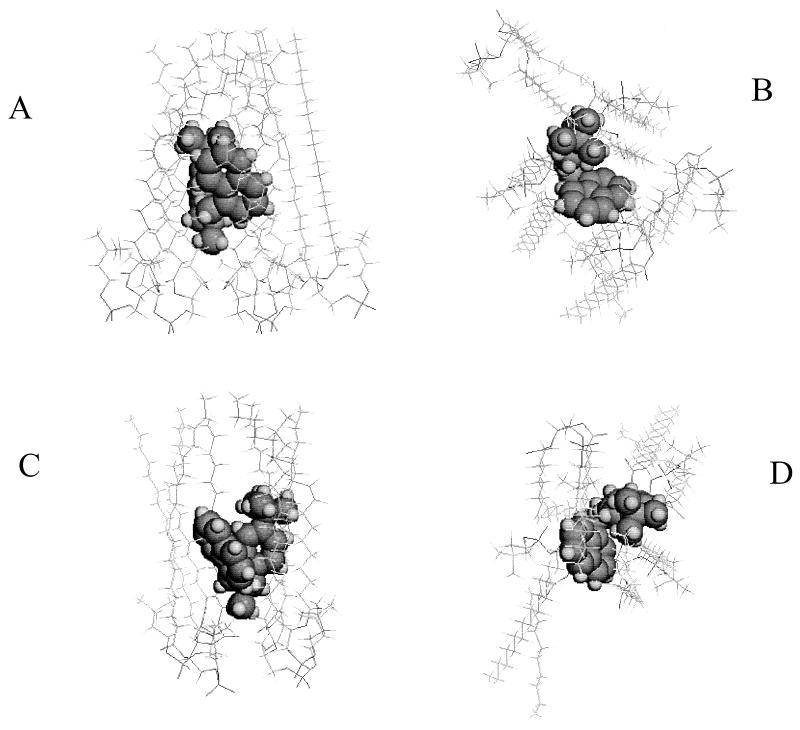
Thereafter, we combined results for the 26 calculated forms of neutral or protonated butenafine with phospholipids at the lipid-water interface plane. Three types of phospholipids were used, POPC (two different isomers are noted, POPC1 and POPC2), DPPC, and DPPE, and each molecule of butenafine was surrounded by a layer of phospholipids. In each combination, butenafine was localized at the level of the phospholipid aliphatic chains close to the polar zone. The methyl group carried by the nitrogen points towards the polar heads of the lipids. Figure 5 shows an example of the interaction between butenafine (R and S enantiomers) and POPC2. Systematic analysis of the interactions among the three forms of butenafine (neutral and R and S enantiomers) and the lipids (POPC1, POPC2, DPPC, and DPPE) had been performed by determining the number of phospholipid molecules in direct interaction with butenafine; the total energy, Et, of the complex; the energy per lipid; and the area occupied at the interface by the phospholipid molecule, taking into consideration the presence or absence of butenafine (Table 1). The results showed that (i) an equal or greater number of phospholipid molecules was necessary to surround the R enantiomer than to surround the neutral form or the S enantiomer; (ii) whatever the structure investigated (the neutral form or the R and S enantiomers), the energy level (Etot/lipid) was less favorable when the drug interacted with DPPE than with DPPC, POPC1, or POPC2; (iii) except for POPC2, the least stable complex was obtained with the R enantiomer; and (iv) interaction with butenafine decreased the interface area of lipids.
FIG. 5.
Assembly of the protonated forms (R [A and B] and S [C and D] enantiomers) with POPC2. (A and C) Lateral views. (B and D) Top views. Butenafine is represented in Corey-Pauling-Koltun (CPK) mode, whereas the phospholipids are represented in skeleton mode.
TABLE 1.
Number of regrouped phospholipids with butenafine, values of internal energy, energy per lipid, and molecular area at the interface with or without butenafine for different assembled forms of the phospholipids POPC1, POPC2, DPPC, and DPPE
| Phospholipid Form | No. of lipid molecules | Etot (kcal/mol) | Etot/lipid (kcal/mol) | Area (Å2) with butenafine | Area (Å2) without butenafine |
|---|---|---|---|---|---|
| POPC1 | |||||
| Neutral | 5 | −218.12 | −43.62 | 51 | 60 |
| R enantiomer | 6 | −165.75 | −27.63 | 60 | 69 |
| S enantiomer | 5 | −206.71 | −41.34 | 49 | 56 |
| POPC2 | |||||
| Neutral | 5 | −239.02 | −47.80 | 58 | 68 |
| R enantiomer | 5 | −237.05 | −47.41 | 58 | 65 |
| S enantiomer | 4 | −259.15 | −64.79 | 55 | 67 |
| DPPC | |||||
| Neutral | 4 | −165.05 | −41.26 | 57 | 69 |
| R enantiomer | 6 | −181.96 | −30.33 | 52 | 57 |
| S enantiomer | 4 | −148.35 | −37.09 | 57 | 70 |
| DPPE | |||||
| Neutral | 5 | −141.87 | −28.37 | 45 | 52 |
| R enantiomer | 6 | −113.27 | −18.88 | 49 | 56 |
| S enantiomer | 6 | −164.12 | −27.35 | 56 | 64 |
DISCUSSION
Butenafine is an allylamine inhibitor of fungal squalene epoxidase that is used in tinea infections. Naftifine, the first representative of this drug class, served as the starting point for intensive studies, which led to the discovery of terbinafine (45, 46). Further structure-activity relationship explorations, concentrating on the allyl side chain, led to the discovery of the homoproparglyamines (43, 53) and the benzylamines (43). Within the latter derivatives, para substitution of the benzyl group is required for high antifungal activity, with butenafine being the preferred molecule (42). In butenafine, the “allylic” double bond is fixed in the E configuration and is an essential feature for maintaining high activity (52, 53).
The mechanism of action and the pharmacokinetic properties of butenafine suggest the importance of interactions between the drug and lipids. When fungal cells are exposed to high concentrations of butenafine (>1,400 μM), large amounts of cations, especially K+, are released, suggestive of a disruption of the cell membrane. Second, the long-lasting effect of butenafine after topical administration is probably related to fungicidal concentrations maintained in the stratum corneum of mammalian skin. In this study, we attempted to characterize the interaction of butenafine with lipids, using both experimental (permeability and fluidity studies) and conformational approaches.
The butenafine concentrations selected for our experimental studies (8 to 800 μg/ml; 25 to 2,500 μM) mirror those found in the epidermis of animals treated with a 1% solution of the drug (250 to 500 μg/g of tissue) (13) and are consistent with those needed to inhibit growth in various yeast strains (0.1 to >100 μg/ml) (46).
Permeability studies were performed with calcein, a relatively large, polar molecule, the release of which probably requires the formation of “pores” through the hydrophobic domain of the membrane. We show here that calcein release is triggered by butenafine. The effect is fast (within 1 min), similar to that observed with diphtheria toxin fragment B (19), the Staphylococcus aureus toxins, leucocidins and γ-hemolysins (20), bacterial carotenoids (23), and gramicidin (48). These data suggest that butenafine is able to grossly perturb membrane integrity and release cell constituents. We therefore confirm and extend the results of Iwatani et al. (27), who showed that Candida albicans cells exposed to butenafine concentrations ranging from 12.5 to 100 μg/ml released large numbers of phosphate ions.
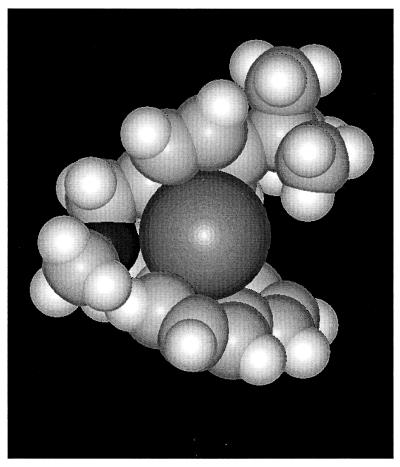
The results from the conformational studies suggest an additional, alternative mechanism by which butenafine may also trigger the release of cations. The different conformations of butenafine calculated in this study indicate that the neutral forms of the drug are appropriate for cation binding. The planes of the two aromatic groups lie at 70° from each other, with their electron cloud orientations facing and forming a “pocket” favorable for cation binding (Fig. 6). The aromatic rings are approximately 3.7 Å apart, while the ionic diameter of potassium is 2.65 Å; moreover, the partial negative charge of the nitrogen atom could play a role in cation retention. This suggests that in addition to an interaction with ion channels, butenafine might facilitate cation transport through the lipid membrane via a mechanism similar to that described for ionophores (8). This direct effect of destabilizing the lipid core of the membrane, however probably occurs at concentrations of butenafine higher than those required for increasing the permeability of K+ channels.
FIG. 6.
Hypothetical scheme for the interaction between the neutral form of butenafine and a potassium ion (dark grey). The nitrogen atom of butenafine is indicated in black.
The results of fluorescence polarization studies using liposomes confirm an interaction between butenafine and lipids. Indeed, butenafine increases the rate of molecular motion of lipids, as shown for other lipophilic drugs, such as bis-(β-diethylaminoethylether) hexestrol (37) and amiodarone (16). Although fluorescence polarization measurements give no information about the fluidity of the individual lipids (32), the interest of the fluorescent probes used here to investigate membrane fluidity is not in doubt. However, the transversal location of DPH and its derivatives in the membrane is still a matter of some controversy. First, DPH was found close to the center of the bilayer in egg phosphatidylcholine vesicles but more broadly distributed in dipalmitoylphosphatidylcholine vesicles (33). Second, although many studies suggested that TMA-DPH essentially probes the glycerol backbone region and the first fatty acyl chain region down to C-8 to C-10 of the lipid (18, 26, 29, 47, 57), a more recent study (28) reports that the absolute change in DPH localization in phosphatidylcholine vesicles upon attachment of anionic or cationic groups (as with TMA-DPH) is relatively small (4 Å). This would suggest that differences in fluorescence polarization of forms of DPH with and without substitutions could reflect a direct effect of substitution on motion rather than an effect on DPH location.
The conformational analysis carried out in this study essentially confirms that butenafine can interact with both the hydrophilic and the hydrophobic domains of the lipid layer. The calculated structures align at the level of the aliphatic chain orienting the nitrogen and the methyl group of butenafine towards the proximity of the polar heads of the phospholipids. Except in one instance and despite the fact that stability depended on the phospholipid type and ionization of the molecule, all the neutral and protonated forms calculated for butenafine were stable in the various phospholipids examined, which is in agreement with the high liposolubility of the molecule (35). Indeed, when protonated, butenafine adopts different conformations according to the proton position on the tertiary amine. For the S enantiomer, the aromatic rings are in close proximity, whereas in the R enantiomer, they are almost in the same plane. In the latter case, charged butenafine stabilizes itself into the different phospholipids according to conformation.
In conclusion, the present study shows that butenafine at high but therapeutically relevant concentrations may exert antifungal activity by interacting with membrane lipids and causing permeabilization of fungal membranes. Insertion of butenafine into membrane lipids, combined with an ability to interact with cations, suggests that an ionophoretic mechanism may also be involved in cation efflux. The fact that the hydrophobicity of butenafine is close to that of squalene, the substrate for squalene epoxidase, suggests that it may fit into the catalytic site for that enzyme. Furthermore, the stable insertion of butenafine into different lipids may provide an explanation for the prolonged half-life of the drug, with lipids in cutaneous tissues acting as a reservoir for the drug, from which it is slowly released. Finally, these results suggest that a physicochemical approach, combined with determinations of biological activity, might be a powerful additional tool for the discovery of novel, potent antifungal drugs with extended duration of action.
ACKNOWLEDGMENTS
We thank Marnie Lett, Didier Lambert, and Paul Depovere for helpful suggestions and Roy Massingham for critical reading of the manuscript. We thank Pascale Segers for her secretarial assistance and the Unité de Pharmacocinétique, Métabolisme, Nutrition and Toxicologie (UCL) for free access to the Perkin-Elmer LS-50 fluorimeter.
R.B., M.-P.M.-L., and F.V.B. are Research Director, Senior Research Associate, and Research Associate of the Belgian Fonds National de la Recherche Scientifique, respectively. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grant 3.4589.96 to M.-P.M.-L.). The financial support of UCB-Pharma is also gratefully acknowledged.
REFERENCES
- 1.Allen T M, Cleland L G. Serum-induced leakage of liposome contents. Biochim Biophys Acta. 1980;597:418–426. doi: 10.1016/0005-2736(80)90118-2. [DOI] [PubMed] [Google Scholar]
- 2.Arika T, Hase T, Yokoo M. Anti-Trichophyton mentagrophytes activity and percutaneous permeation of butenafine in guinea pigs. Antimicrob Agents Chemother. 1993;37:363–365. doi: 10.1128/aac.37.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arika T, Yokoo M, Hase T, Maeda T, Amemiya K, Yamaguchi H. Effects of butenafine hydrochloride, a new benzylamine derivative, on experimental dermatophytosis in guinea pigs. Antimicrob Agents Chemother. 1990;34:2250–2253. doi: 10.1128/aac.34.11.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett G R. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 5.Bradshaw J P, Darkes M J M, Katsaras J, Epand R M. Neutron diffraction studies of viral peptides. Physica B. 2000;276:495–498. [Google Scholar]
- 6.Brasseur R. Calculation of the three-dimensional structure of Saccharomyces cerevisae cytochrome b inserted in a lipid matrix. J Biol Chem. 1988;263:12571–12575. [PubMed] [Google Scholar]
- 7.Brasseur R, editor. Molecular description of biological membrane components by computer-aided conformational analysis. I and II. Boca Raton, Fla: CRC Press; 1990. [Google Scholar]
- 8.Brasseur R, Ruysschaert J-M. Conformation and mode of organization of amphiphilic membrane components: a conformational analysis. Biochem J. 1986;238:1–11. doi: 10.1042/bj2380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasseur R, Deleers M, Malaisse W J, Ruysschaert J M. Conformational analysis of the calcium-A23187 complex at a lipid-water interface. Proc Natl Acad Sci USA. 1982;79:2895–2897. doi: 10.1073/pnas.79.9.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasseur R, Goormaghtigh E, Ruysschaert J M. Theoretical conformational analysis of phospholipid bilayers. Biochem Biophys Res Commun. 1981;103:301–310. doi: 10.1016/0006-291x(81)91693-4. [DOI] [PubMed] [Google Scholar]
- 11.Brasseur R, Killian J A, De Kruijff B, Ruysschaert J M. Conformational analysis of gramicidin-gramicidin interactions at the air-water interface suggests that gramicidin aggregates into tube-like structures similar as [sic] found in the gramicidin-induced hexagonal HII phase. Biochim Biophys Acta. 1987;903:11–17. doi: 10.1016/0005-2736(87)90150-7. [DOI] [PubMed] [Google Scholar]
- 12.Brasseur R, Vandenbranden M, Cornet B, Burny A, Ruysschaert J M. Orientation into the lipid bilayer of an asymmetric amphipathic helical peptide located at the N-terminus of viral fusion proteins. Biochim Biophys Acta. 1990;1029:267–273. doi: 10.1016/0005-2736(90)90163-i. [DOI] [PubMed] [Google Scholar]
- 13.Brennan B, Leyden J J. Overview of topical therapy for common superficial fungal infections and the role of new topical agents. Am Acad Dermatol. 1997;36:S3–S8. doi: 10.1016/s0190-9622(97)70315-3. [DOI] [PubMed] [Google Scholar]
- 14.Buldt G, Gally H U, Seelig A, Seelig J, Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978;271:182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- 15.Chatelain P, Brasseur R. Conformational analysis of cardiovascular drug-lipid interactions: propanolol and amiodarone. In: Brasseur R, editor. Molecular description of biological membranes by computer-aided conformational analysis. II. Mode of insertion of amphiphilic components into the lipid membrane. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 43–62. [Google Scholar]
- 16.Chatelain P, Ferreira J, Laruel R, Ruysschaert J M. Amiodarone induced modifications of the phospholipid physical state. A fluorescence polarization study. Biochem Pharmacol. 1986;35:3007–3013. doi: 10.1016/0006-2952(86)90379-5. [DOI] [PubMed] [Google Scholar]
- 17.Chiang C C, Paul I C. Monomeric forms of the acid ionophore lasalocid A (X-537A) from polar solvents. Science. 1977;196:1441–1443. doi: 10.1126/science.867039. [DOI] [PubMed] [Google Scholar]
- 18.Davenport L, Dale R E, Bisby R H, Cundall R B. Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985;24:4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- 19.Defrise-Quertain F, Cabiaux V, Vandenbranden M, Wattiez R, Falmagne P, Ruysschaert J M. pH-dependent bilayer destabilization and fusion of phospholipidic large unilamellar vesicles induced by Diphtheria Toxin and its fragments A and B. Biochemistry. 1989;28:3406–3413. doi: 10.1021/bi00434a040. [DOI] [PubMed] [Google Scholar]
- 20.Ferreras M, Hoper F, Dalla Serra M, Colin D A, Prévost G, Menestrina G. The interaction of Staphylococcus aureus bi-component gamma-hemolysins and leucocidins with cells and lipid membranes. Biochem Biophys Acta. 1998;1414:108–126. doi: 10.1016/s0005-2736(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 21.Georgopapadakou N H. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Mirobiol. 1998;1:547–557. doi: 10.1016/s1369-5274(98)80087-8. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A K, Einarson T R, Summerbell R C, Shear N H. An overview of topical antifungal therapy in dermatomycoses. Drugs. 1998;55:645–674. doi: 10.2165/00003495-199855050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hara M, Yuan H, Yang Q, Hoshino T, Yokoyama A, Miyake J. Stabilization of liposomal membranes by thermozeaxanthins:carotenoid-glucide esters. Biochim Biophys Acta. 1999;1461:147–154. doi: 10.1016/s0005-2736(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 24.Hiratani T, Asagi Y, Yamaguchi H. Studies on antifungal mechanism of action of butenafine hydrochloride. I. Inhibition of squalene epoxidation and damaging cell membranes in Sporothrix schenckii cells. Jpn J Med Mycol. 1991;32:139–149. [Google Scholar]
- 25.Hiratani T, Asagi Y, Yamaguchi H. Studies on antifungal mechanism of action of butenafine hydrochloride. II. Comparison in the response to drug treatment between a wild-type strain and tolciclate-resistant mutant strains of Sporothrix schenckii. Jpn J Med Mycol. 1991;32:151–157. [Google Scholar]
- 26.Ho C, Slater S J, Stubbs C D. Hydration and order in lipid bilayers. Biochemistry. 1995;34:6188–6195. doi: 10.1021/bi00018a023. [DOI] [PubMed] [Google Scholar]
- 27.Iwatani W, Arika T, Yamaguchi H. Two mechanisms of butenafine action in Candida albicans. Antimicrob Agents Chemother. 1993;37:785–788. doi: 10.1128/aac.37.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaiser R D, London E. Localisation of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry. 1998;37:8180–8190. doi: 10.1021/bi980064a. [DOI] [PubMed] [Google Scholar]
- 29.Kitagawa S, Matsubayashi M, Kotani K, Usui K, Kametani F. Asymmetry of membrane fluidity in the lipid bilayer of blood platelets: fluorescence study with diphenylhexatriene and analogs. J Membr Biol. 1991;119:221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- 30.Laurent G, Carlier M B, Rollman B, Van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31:3861–3870. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 31.Lelkes P I. Methodological aspects dealing with stability measurements of liposomes in vitro using the carboxyfluorescein assay. In: Gregoriadis G, editor. Liposome technology. Boca Raton, Fla: CRC Press; 1984. pp. 225–246. [Google Scholar]
- 32.Lentz B R. Membrane fluidity as detected by diphenylhexatriene probes. Chem Phys Lipids. 1989;50:171–190. [Google Scholar]
- 33.Lentz B R. Use of fluorescence probes to monitor molecular order and motions within liposome bilayers. Chem Phys Lipids. 1993;64:99–116. doi: 10.1016/0009-3084(93)90060-g. [DOI] [PubMed] [Google Scholar]
- 34.Lesher J L, Jr, Babel D E, Stewart D M, Jones T M, Kaminester L, Goldman M, Weintraub J S. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehicle-controlled, double-blind trial. J Am Acad Dermatol. 1997;36:S20–S24. doi: 10.1016/s0190-9622(97)70318-9. [DOI] [PubMed] [Google Scholar]
- 35.Maeda T, Takase M, Ishibashi A, Yamamoto T, Sasaki K, Arika T, Yokoo M, Amemiya K. Synthesis of a new benzylamine antifungal agent butenafine hydrochloride (KP-363) and its antifungal activity. Pharmacol J. 1991;111:126–138. doi: 10.1248/yakushi1947.111.2_126. [DOI] [PubMed] [Google Scholar]
- 36.McNeely W, Spencer C M. Butenafine. Drugs. 1998;55:405–412. doi: 10.2165/00003495-199855030-00006. [DOI] [PubMed] [Google Scholar]
- 37.Mingeot-Leclercq M P, Schanck A, Ronveaux-Dupal M F, Deleers M, Brasseur R, Ruysschaert J M, Laurent G, Tulkens P M. Ultrastructural, physico-chemical and conformational study of the interactions of gentamicin and bis(beta-diethylaminoethylether)hexestrol with negatively charged phospholipid bilayers. Biochem Pharmacol. 1989;38:729–741. doi: 10.1016/0006-2952(89)90225-6. [DOI] [PubMed] [Google Scholar]
- 38.Montenez J P, Van Bambeke F, Piret J, Brasseur R, Tulkens P M, Mingeot-Leclercq M P. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol Appl Pharmacol. 1999;156:129–140. doi: 10.1006/taap.1999.8632. [DOI] [PubMed] [Google Scholar]
- 39.Montenez J P, Van Bambeke F, Piret J, Schanck A, Brasseur R, Tulkens P M, Mingeot-Leclercq M P. Interaction of the macrolide azithromycin with phospholipids. II. Biophysical and computer-aided conformational studies. Eur J Pharmacol. 1996;314:215–227. doi: 10.1016/s0014-2999(96)00553-5. [DOI] [PubMed] [Google Scholar]
- 40.Mouritsen O G, Jorgensen K. Dynamical order and disorder in lipid bilayers. Chem Phys Lipids. 1994;73:3–25. doi: 10.1016/0009-3084(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 41.Nelder J A, Mead R. A simplex method for function minimization. Comput J. 1965;7:308–313. [Google Scholar]
- 42.Nussbaumer P, Dorfstütter G, Grassberger M A, Leitner I, Meingassner J G, Thirring K, Stutz A. Synthesis and structure-activity relationships of Phenyl-substituted Benzylamine antimycotics: a novel Benzylbenzylamine antifungal agent for systemic treatment. J Med Chem. 1993;36:2115–2120. doi: 10.1021/jm00067a010. [DOI] [PubMed] [Google Scholar]
- 43.Nussbaumer P, Petranyi G, Stutz A. Synthesis and structure-activity relationships of benzo[b] thienylallylamine antimycotica. J Med Chem. 1991;34:65–73. doi: 10.1021/jm00105a011. [DOI] [PubMed] [Google Scholar]
- 44.Odom R B. Update on topical therapy for superficial fungal infections: focus on butenafine. Am Acad Dermatol. 1997;36:S1–S2. doi: 10.1016/s0190-9622(97)70314-1. [DOI] [PubMed] [Google Scholar]
- 45.Petranyi G, Meingassner J G, Mieth H. Activity of terbinafine in experimental fungal infections of laboratory animals. Antimicrob Agents Chemother. 1987;31:1558–1561. doi: 10.1128/aac.31.10.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petranyi G, Meingassner J G, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987;31:1365–1368. doi: 10.1128/aac.31.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prendergast F G, Haugland R P, Callahan P J. TMA-DPH, synthesis, fluorescence properties, and use as fluorescence probe of lipid bilayers. Biochemistry. 1981;20:7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- 48.Prenner E J, Lewis R N A, Jelokhani-Niaraki M, Hodges R S, McElhaney R N. Cholesterol attenuates the interaction of the antimicrobial peptide gramicidin S with phospholipid bilayer membranes. Biochem Biophys Acta. 2001;1510:83–92. doi: 10.1016/s0005-2736(00)00337-0. [DOI] [PubMed] [Google Scholar]
- 49.Rahman M, Brasseur R. WinMGM: a fast CPK molecular graphics program for analyzing molecular structure. J Mol Graphics. 1994;12:212–218. doi: 10.1016/0263-7855(94)80090-1. [DOI] [PubMed] [Google Scholar]
- 50.Savin R, De Villez R L, Elewski B, Hong S, Jones T, Lowe N, Lucky A, Reyes B, Willis I. One-week therapy with twice-daily butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Am Acad Dermatol. 1997;36:S15–S19. doi: 10.1016/s0190-9622(97)70317-7. [DOI] [PubMed] [Google Scholar]
- 51.Shinitzky M, Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicethylphosphate. J Biol Chem. 1974;249:2652–2657. [PubMed] [Google Scholar]
- 52.Stutz A. Synthesis and structure-activity correlations within allylamine antimycotics. Ann N Y Acad Sci. 1988;544:46–62. doi: 10.1111/j.1749-6632.1988.tb40388.x. [DOI] [PubMed] [Google Scholar]
- 53.Stutz A, Georgopoulos A, Granitzer W, Petranyi G, Berney D. Synthesis and structure-activity relationships of naftifine-related allylamine. J Med Chem. 1986;29:112–125. doi: 10.1021/jm00151a019. [DOI] [PubMed] [Google Scholar]
- 54.Tschen E, Elewski B, Gorsulowsky D C, Pariser D M. Treatment of interdigital tinea pedis with a 4-week once-daily regimen of butenafine hydrochloride 1% cream. J Am Acad Dermatol. 1997;36:S9–S14. doi: 10.1016/s0190-9622(97)70316-5. [DOI] [PubMed] [Google Scholar]
- 55.Tulkens P M, Mingeot-Leclercq M P, Laurent G, Brasseur R. Conformational and biochemical analysis of the interactions between phospholipids and aminoglycoside antibiotics in relation with their toxicity. In: Brasseur R, editor. Molecular description of biological membrane components by computer-aided conformational analysis. Boca Raton, Fla: CRC Press; 1990. pp. 63–93. [Google Scholar]
- 56.Weinstein J N, Yoshikami S, Henkart P, Blumenthal R, Hagins W A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977;195:489–491. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- 57.Zicha J, Kunes J, Le Quan Sang K H, Devynck M A. Regulation of the dynamic properties of platelet plasma membrane by intracellular sodium ions. Life Sci. 1993;52:1559–1565. doi: 10.1016/0024-3205(93)90056-9. [DOI] [PubMed] [Google Scholar]