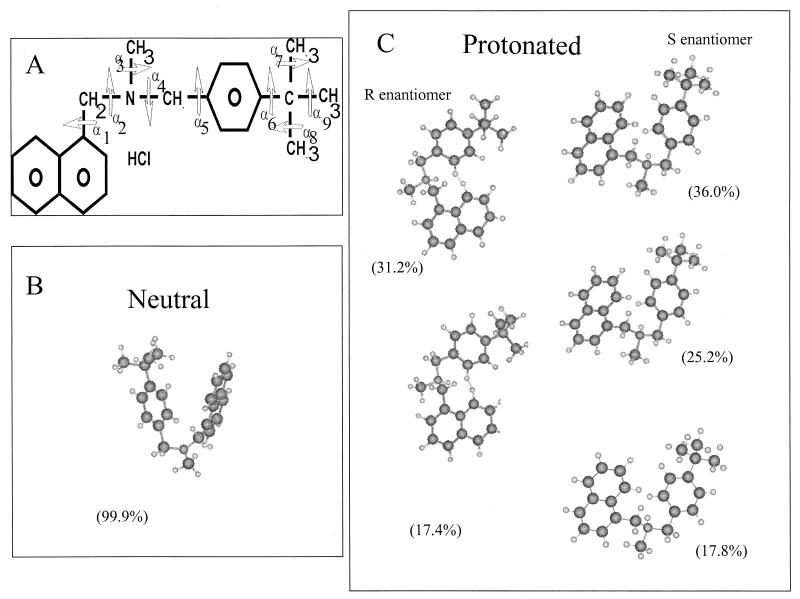
FIG. 3.
(A) Primary structure of butenafine; the torsion angles are annotated α1 to α9. (B and C) Selected structures of butenafine (probability, >15%) obtained by the Simplex energetic minimization procedure. The percentages of probability of the neutral form (B) and the protonated forms (R and S enantiomers) (C) are indicated for each structure.