Table 1.
Summary of the main characteristics of representative ORR redox mediators in aprotic Li–O2 batteries.
| RM | Electrolyte | Cathode | Current density | Discharge capacity |
|---|---|---|---|---|
| DBBQ [9] | 10 mM DBBQ + 1.0 M LiTFSI in TEGDME | GDL | 0.1 mA cm–2 | 436 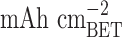
|
| Q10 [20] | 10 mM Q10 + 1.0 M LiTFSI in TEGDME | Super P | 0.1 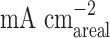
|
575 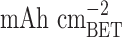
|
| BDTD [24] | 20 mM BDTD + 1.0 M LiTFSI in TEGDME | CNT | 0.1 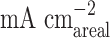
|
4.7 mAh cm–2 |
| VK2 [21] | 10 mM VK2 + 1.0 M LiTFSI in DME | GDL | 0.09 mA cm–2 | 3.6 mAh cm–2 |
 [22]
[22] |
50 mM 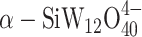 +1.0 M LiTFSI in DMSO +1.0 M LiTFSI in DMSO |
Carbon cloth | 0.1 uA cm–2 | 0.6 mAh cm–2 |
| TTM [25] | Saturated TTM + 1.0 M LiOTF in TEGDME | Super P | 0.1 mA cm–2 | 7.5 mAh cm–2 |
| 1,8 DNAQ [23] | 10 mM 1,8 DNAQ + 0.5 M LiTFSI in TEGDME | Carbon paper | 0.1 mA cm–2 | 2.25 mAh cm–2 |
GDL, gas diffusion layer; BDTD, benzo[1,2-b:4,5-b']dithiophene-4,8-dione; CNT, carbon nanotubes; VK2, vitamin K2; TTM, tris(2,4,6-trichlorophenyl)methyl.
