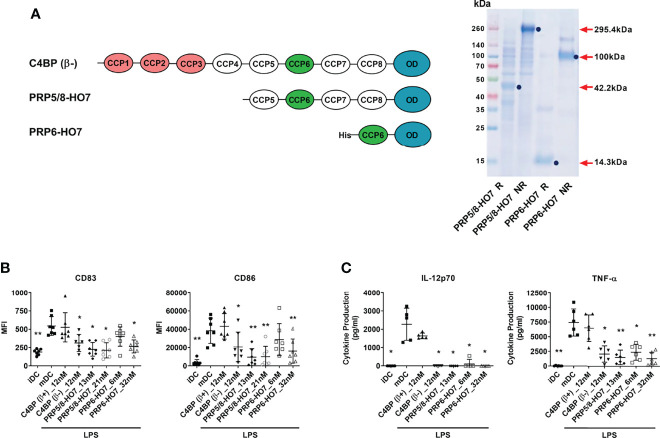
Figure 2.
Deletion of the complement inhibitory domains of C4BP(β-) does not affect its immunomodulatory activity. (A) Left: Schematic structure of the α-chains from C4BP(β−), and from its variants PRP5/8-HO7 and PRP6-HO7. The CCP1-CCP3 complement inhibitory domains are depicted in red. The CCP6 immunomodulatory domain is depicted in green. The oligomerization domain (OD) is depicted in blue. “His” refers to a poly-histidine tag located at the N-terminus of PRP6-HO7. Right: SDS-PAGE and Coomassie Blue staining of both PRP5/8-HO7 and PRP6-HO7 under reducing (R) and non-reducing (NR) conditions. Red arrows and blue dots indicate the location and size of both reduced and non-reduced protein forms. Left lane, molecular weight marker. (B) Human Mo-DCs were incubated throughout their differentiation process with C4BP(β+), C4BP(β-) (both at 12 nM) and the variants PRP5/8-HO7 and PRP6-HO7 at the indicated concentrations. DC maturation was achieved by LPS treatment. Cells were then collected, washed, and analyzed by flow cytometry for cell surface expression of the activation marker CD83 and the co-stimulatory molecule CD86. MFI, median fluorescence intensity for the different surface markers. (C) The concentrations of IL-12p70 and TNF-α were analyzed in the respective cell supernatants by ELISA. iDC, untreated, immature DCs; mDC, untreated, LPS-matured DCs. The results shown are the mean ± SD from 7 independent donors (cell surface markers), or from 5-6 independent donors performed in duplicate (cytokines) (*p < 0.05; **p < 0.01 compared with mDC).