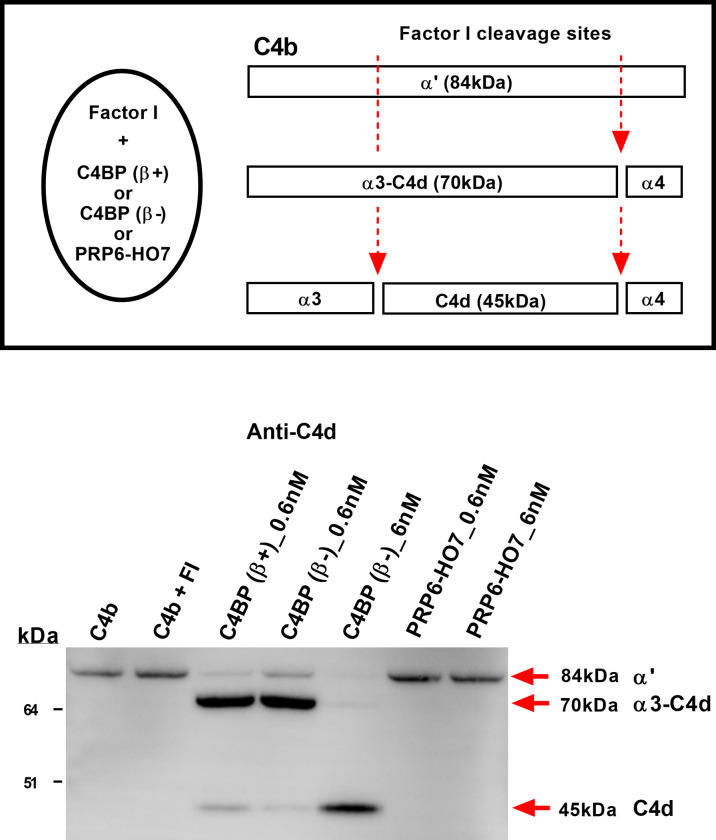
Figure 4.
PRP6-HO7 is devoid of complement inhibitory activity. Schematic drawing of C4BP(β+) and C4BP(β-) cofactor activity for factor I-mediated splitting of C4b. Factor I cleaves the α′-chain of C4b at two sites. Partial cleavage generates fragments α3-C4d (70 kDa) and α4 (14 kDa). Further cleavage of α3-C4d yields the small C4d (45 kDa) fragment which remains associated with targets. C4BP(β+), C4BP(β-) and PRP6-HO7, at the indicated concentrations (lanes 3-7), were incubated with C4b (8.9 μg/ml) followed by addition of factor I (4.4 μg/ml). Reaction controls included C4b alone, and C4b + FI. All reactions were stopped after 30 min with SDS-reducing sample buffer. C4b cleavage fragments were separated by 4-12% SDS-PAGE under reducing conditions followed by Western blotting using an anti-C4d MoAb. Red arrows indicate the size of the C4b fragments. Cofactor activity was confirmed by the appearance of α3-C4d (70 kDa) or C4d (45 kDa). Results are representative of 3 independent experiments.