Abstract
Lipid nanoparticles (LNPs) can be used as delivery vehicles for nucleic acid biotherapeutics. In fact, LNPs are currently being used in the Pfizer/BioNTech and Moderna COVID-19 vaccines. Cationic LNPs composed of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/cholesterol (chol) LNPs have been classified as one of the most efficient gene delivery systems and are being tested in numerous clinical trials. The objective of this study was to examine the effect of the molar ratio of DOTAP/chol, PEGylation, and lipid to mRNA ratio on mRNA transfection, and explore the applications of DOTAP/chol LNPs in pDNA and oligonucleotide transfection. Here we showed that PEGylation significantly decreased mRNA transfection efficiency of DOTAP/chol LNPs. Among non-PEGylated LNP formulations, 1:3 molar ratio of DOTAP/chol in DOTAP/chol LNPs showed the highest mRNA transfection efficiency. Furthermore, the optimal ratio of DOTAP/chol LNPs to mRNA was tested to be 62.5 µM lipid to 1 μg mRNA. More importantly, these mRNA-loaded nanoparticles were stable for 60 days at 4 °C storage without showing reduction in transfection efficacy. We further found that DOTAP/chol LNPs were able to transfect pDNA and oligonucleotides, demonstrating the ability of these LNPs to transport the cargo into the cell nucleus. The influence of various factors in the formulation of DOTAP/chol cationic LNPs is thus described and will help improve drug delivery of nucleic acid–based vaccines and therapies.
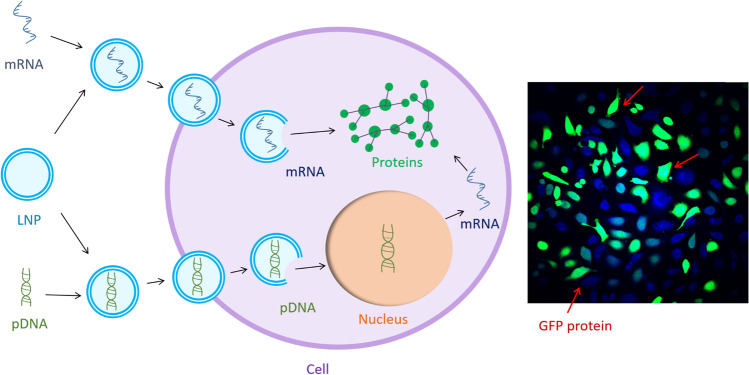
Graphical abstract
KEY WORDS: lipid nanoparticles, mRNA delivery, DOTAP/cholesterol, LNP-mRNA, transfection efficiency
INTRODUCTION
The development of nucleic acid biotherapeutics highly relies on a safe and efficient delivery system to protect the cargo from degradation and to deliver it to the target cells for efficient uptake. Nucleic acid delivery systems can be broadly classified into viral or nonviral vectors. Compared to viral vectors, nonviral vectors possess many advantages particularly with respect to safety because they tend to have less toxicity and immunogenicity (1, 2). Cationic lipoplexes, complexes composed of synthetic positively charged lipids and negatively charged nucleic acid, are the most widely used nonviral delivery system for nucleic acid drugs. Even though many cationic lipid–based vectors have been shown to perform extremely efficiently in cellular transfection in vitro, they show limited function when tested in animals (3, 4). DOTAP/chol-based delivery vehicles, however, are promising for transporting nucleic acids in cells in vivo (5–7), possibly due to the role of chol in improving the stability and efficiency of chol-containing LNPs in serum conditions (8, 9). In addition, the preparation of these lipid nanoparticles for clinical use has been refined and is FDA approved (10).
Even though DOTAP/chol LNPs have been extensively studied regarding their ability to transport plasmid DNA (pDNA) and antisense oligonucleotide to cells (11–13), detailed investigation needs to be done to achieve more comprehensive guidelines for optimal performance. First, a thorough investigation on mRNA delivery by DOTAP/chol LNPs is beneficial to the development of potential mRNA vaccines and therapies. Compared to DNA, although mRNA is less stable, it causes fewer immune responses (2). Moreover, since mRNA is directly delivered and expressed in the cytoplasm without requiring nuclear localization for expression, there is no potential for mutagenesis due to genomic integration (14, 15). Next, the molar ratio of cationic lipid to neutral lipid usually plays an important part in determining the optimal transfection efficiency for many liposomal formulations (16, 17). Satisfactory transfection efficiencies of DOTAP/chol lipoplexes with molar ratios of 1:1, 2:1, or 3:1 have been reported in different studies (18–20). Since the RNAs they used to complex with DOTAP/chol LNPs were different, so were their treatment methods and cell lines, it is difficult to conclude which molar ratio will present the optimal transfection efficiency. Hence, we aimed to test the efficacy of DOTAP/chol LNPs at different molar ratios under the same experimental conditions for better comparison. Based on this, we further expanded the molar ratio range to include 1:2, 1:3, and 1:4 to incorporate more chol in the formulation. Studying higher proportions of chol is necessary as it has been indicated that as chol content increases, cationic lipoplexes show less toxicity, more stability in serum conditions, and higher transfection ability (20, 21). Secondly, PEGylation (surface modification by attaching polyethylene glycol) also plays a major role in determining the best transfection ability. Although PEGylation has been shown to improve the stability and additionally prolong the systemic circulation time of lipoplexes (22), some adverse effects have been described to limit the cellular internalization and endosomal escape of lipid nanoparticles, eventually leading to much-lower transfection efficiency (23, 24). Therefore, analyzing the effect of PEGylation on the transfection ability of DOTAP/chol lipoplexes in serum would prove useful for in vivo applications. Lastly, understanding how the ratio between lipid concentration and nucleic acids affects transfection efficiency is necessary, as different conclusions have been drawn regarding this matter, including higher charge induced by more lipid promotes transfection efficiency (25), or there exists a middle value of lipid/nucleic acids to achieve the highest transfection efficiency (26). As a result, it is essential to examine how this factor affects DOTAP/chol LNPs.
In this study, we comprehensively analyzed the effects of various factors on the mRNA transfection efficiency of DOTAP/chol LNPs and lipoplexes, including PEGylation, DOTAP to chol molar ratio, and lipid to mRNA ratio, to develop a non-invasive mRNA carrier with a higher transfection efficiency. Additionally, we explored the possibility of DOTAP/chol LNPs to deliver pDNA and oligonucleotide (a single-stranded DNA) for the purpose of creating a formulation with multiple applications in vaccine development and disease management.
MATERIALS AND METHODS
Materials
1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethyleneglycol)-2000] (DSPE-PEG-2000) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Chol, chloroform, McCoy’s 5a modified growth media, and fetal bovine serum were purchased from Millipore Sigma (St. Louis, MO). The Aqueous One Solution Cell Proliferation Assay (MTS) was obtained through Promega (Madison, WI). The 5 × DNA loading buffers were purchased from Thomas Scientific (Swedesboro, NJ). The single-stranded fluorescent-labeled oligonucleotide 5′–[6-FAM] CTCCTCCCATTTTTATAAG–3′ (27) was obtained from Eurofins MWG Operon (Huntsville, AL). SK-OV-3 cell line was purchased from the American Type Culture Collection (Manassas, VA). JetPRIME® (JP) reagent for mRNA transfection was purchased from Polyplus Transfection (New York, NY). Effectene transfection reagent (obtained from Qiagen, Germantown, MD) and plasmid DNA were kindly gifted to us by Dr. Mohammad Ali (Binghamton University).
Preparation of Blank LNPs and LNP-mRNA/DNA Complexes (Lipoplexes)
PEGylated or non-PEGylated DOTAP/chol LNPs were prepared by the thin-film evaporation method. Briefly, DOTAP/chol at molar ratios of 2:1, 1:1, 1:2, 1:3, and 1:4, with or without 2.5% DSPE-PEG-2000, were dissolved in 2 mL chloroform in a round bottom flask to a total amount of 10 µmol, and the solvent was then evaporated under a vacuum by rotary evaporation for the generation of a thin lipid film. A total of 1 mL of 10 mM phosphate-buffered saline (pH = 7.4) was added to the film which was subjected to 30 s sonicating (Branson Ultrasonics Corporation, Danbury, CT, USA) and 60 s vortexing cycles at room temperature until the film was completely hydrated and the solution was homogeneous. The multilamellar liposomal solution was extruded through a 200-nm and then a 100-nm polycarbonate membrane 10 times using a manual extruder from Avanti Polar Lipid (Alabaster, AL). The cationic LNPs were then complexed to anionic mRNA (1 µg), oligonucleotide (1 µg), or pDNA (0.3 µg) by spontaneous electrostatic interaction for 30 min at room temperature.
Characterization of Blank LNPs and Lipoplexes
A Zetasizer Nano (Malvern Instruments, Worcestershire, UK) was used to investigate the particle size distribution and zeta potential of the blank LNPs (62.5 µM lipid) and lipoplexes (62.5 µM lipid complexed with 1 µg mRNA or 0.3 µg pDNA) using the dynamic light scattering technique. Briefly, 100 μL prepared nanoparticle suspension and 900 μL ultrapure water were mixed and then added to a 1-mL cuvette. All measurements were done in triplicate.
Gel Retardation Assay
The mRNA binding affinities of DOTAP/chol LNPs were determined by electrophoresing lipoplexes in a agarose gel (2%) containing 10 μL Gel Green stain in TAE (Tris/Acetic/EDTA) buffer for 30 min at 80 V. Briefly, LNPs with different DOTAP/chol molar ratios (2:1, 1:1, 1:2, 1:3, and 1:4) at various concentrations (31.25, 62.5, and 125 μM) were complexed with a fixed amount of mRNA (1 μg) to make lipoplex suspensions (each sample has a total volume of 15 μL). Then, the suspensions were mixed with 5 μL of 5 × DNA loading buffer. The DNA ladder was used as a marker and naked mRNA (1 μg) was used as a negative control. The resulting mRNA migration pattern was revealed under UV irradiation with an Azure Imaging System (Dublin, CA).
Cell Viability Assays
All cell studies were carried out in a humidified 37 °C, 5% CO2 (standard conditions) atmosphere incubator. Human ovarian cancer SK-OV-3 cells were cultured with McCoy’s 5a modified growth media supplemented with 10% fetal bovine serum (FBS) and 1 × penicillin–streptomycin. PEGylated or non-PEGylated DOTAP/chol at 31.25, 62.5, and 125 µM lipid concentrations with DOTAP/chol molar ratios of 2:1, 1:1, 1:2, 1:3, and 1:4, and a phosphate buffer saline (PBS) control group, were tested in the cell line. The cells were seeded at 5 × 103 cells/well (100 µL/well) in 96-well plates and allowed to grow for 24 h. After this, the medium was removed and replaced with 100 µL of medium containing the blank LNP samples. Following a 24-h incubation period, the old media were removed and replaced with 100 µL fresh media containing 20 µL of MTS solution. The UV–Vis absorbance was read at 490 nm after 2 h of incubation. This viability tests were repeated three times with three internal replicates.
In Vitro mRNA, pDNA, and Oligonucleotide Transfection
For transfection efficiency analysis, SK-OV-3 cells were seeded in 12-well plates with a density of 1 × 105 cells per well overnight. DOTAP/chol LNPs at different molar ratios and concentrations (3.13 μL for 31.25 μM, 6.25 μL for 62.5 μM, 12.5 μL for 125 μM) were incubated with either GFP-expressing mRNA (1 μg), GFP-expressing pDNA (0.3 μg), or fluorescence-labeled oligonucleotide (1 μg) for 30 min to form lipoplexes. Then, PBS was added in these lipoplex suspensions to make their volume equal at 100 μL and added to the cells with 900 μL complete growth media (McCoy’s 5A with 10% FBS). Twenty-four hours (mRNA transfection) or 48 h (pDNA transfection) later, the cells were trypsinized for 5 min and then collected with complete growth media. After centrifuging the cells at 3000 rcf for 5 min, the supernatant was discarded and the cell pellet was collected, resuspended in PBS, and centrifuged again at 3000 rcf for 5 min. Then, the cell pellet was collected and resuspended in PBS for flow cytometry (Becton–Dickinson, San Jose, CA) analysis. Transfection using JetPRIME or Effectene reagent as positive control for mRNA or pDNA transfection was performed according to the manufacturers’ standard protocols. The mRNA and pDNA transfection efficiency was determined by flow cytometry (Becton–Dickinson, San Jose, CA, USA) as the percentage of the GFP-expressing cells against all cells counted. For oligonucleotide transfection, after a 24-h treatment, we processed the cells and harvested the nuclei. This is because this oligonucleotide only functions inside cell nuclei, and since it is fluorescence-labeled, testing the fluorescence intensity shows whether the oligonucleotide enters the nucleus. Briefly, the cells were washed with cold PBS twice, and resuspended in hypotonic buffer in prechilled microcentrifuge tubes. After incubation on ice for 30 min with gentle agitation, the cells were centrifuged at 9000 rcf for 10 min at 4 °C. The supernatant (cytoplasm) was discarded and the pellet was collected and resuspended in PBS for transfection analysis using FCM. The transfection efficiency was determined as the percentage of the fluorescent cell nuclei against all nucleic counted. The integrated stimulated Raman scattering (SRS) and two-photon excitation fluorescence (TPEF) microscopy system was used to visualize mRNA transfection with the same settings as previously described (16). Briefly, cells were imaged by the label-free SRS at 2930 cm−1 and the GFP-transfected cells were visualized by the TPEF simultaneously.
Statistical Analysis
Linear models with post hoc contrasts and multiple testing correction (Dunnett correction) when appropriate were used for statistical comparisons. Correlations were assessed with Spearman correlation coefficients. An alpha of 0.05 was pre-specified.
RESULTS
Characterization of DOTAP/chol LNPs and Lipoplexes
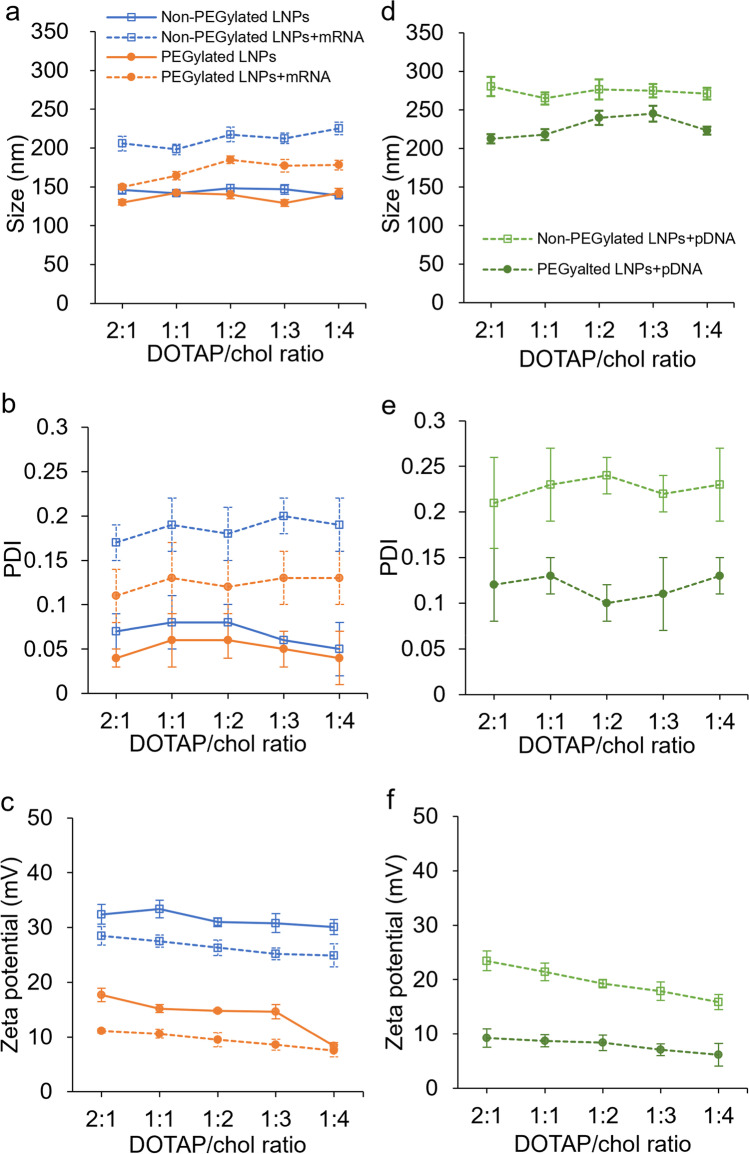
The size, ζ-potential, and polydispersity indexes of blank LNPs and lipoplexes were examined using dynamic light scattering technique. As shown in Fig. 1, differences were observed in the particle size, PDI, and ζ-potential between LNPs and lipoplexes. Following the complexation with mRNA or pDNA, the mean particle size (both p < 0.0001; Fig. 1a, d) and PDI (both p < 0.0001; Fig. 1b, e) increased, while the ζ-potential (both p < 0.0001; Fig. 1c, f) of the complexes decreased. This indicated that adding a negatively charged large molecule such as mRNA and pDNA to the lipid-based particles might induce large, less uniformly distributed complexes compared to blank LNPs. PEG coating also influences the physical characteristics of the prepared liposomal formulations. LNPs with PEGylation exhibited reduced average size (p = 0.034 for blank LNPs; p < 0.0001 when complexed with mRNA or pDNA) compared to the ones without PEGylation. As for PDI and surface charge, PEGylated LNPs showed smaller PDI (p = 0.037 for blank LNPs; p < 0.0001 when complexed with pDNA or mRNA) and ζ-potential (all p < 0.0001) compared to non-PEGylated LNPs. Furthermore, correlations between DOTAP/chol molar ratio and the ζ-potential can be observed in Fig. 1c and f in that the surface charge decreased as the amount of the cationic lipid DOTAP decreased in the formulation (Spearman correlations for combinations of blank LNPs, LNPs complexed with mRNA or pDNA, and PEGylation status ranging between 0.58 and 0.92).
Fig. 1.
The size (a), PDI (b), and zeta potential (c) of non-PEGylated and PEGylated DOTAP/chol LNPs and LNPs/mRNA complexes; the size (d), PDI (e), and zeta potential (f) of non-PEGylated and PEGylated DOTAP/chol LNPs and LNPs/pDNA complexes. Data are presented as mean ± SD (n = 4)
mRNA Binding Affinity of DOTAP/chol Lipoplexes
A gel retardation assay was performed to test the mRNA binding affinity of these lipoplexes with different DOTAP/chol molar ratios and lipid concentrations. Figure 2a showed no free mRNA migration in the gel, meaning complete binding was achieved for all the non-PEGylated formulations regardless of DOTAP/chol molar ratio and lipid concentration. While for the PEGylated formulations, a free mRNA migration path was observed at the lowest lipid concentration (31.25 µM) with a DOTAP/chol molar ratio of 1:4. Based on this assay, PEGylation might decrease the mRNA binding affinity when the overall amount of lipid as well as the cationic lipid DOTAP in the formulation is low, but further research is warranted to confirm the observed relationships.
Fig. 2.
mRNA gel retardation assays of a non-PEGylated DOTAP/chol lipoplexes and b PEGylated DOTAP/chol lipoplexes. DOTAP/chol LNPs were complexed with mRNA at various lipid concentrations, and then run through a 2% agarose gel. The mobility of mRNA was visualized by ethidium bromide staining. The amount of mRNA was fixed at 1 μg
Cytotoxicity of DOTAP/chol LNPs in SK-OV-3 Cells
Three variables were examined in SK-OV-3 cells to determine cytotoxic effects: DOTAP/chol molar ratio, lipid concentration, and PEGylation. As shown in Fig. 3, for both PEGylated and non-PEGylated nanoparticles with the same lipid concentration, a decrease in DOTAP/chol ratio (from 2:1 to 1:4) led to improved cell survival (Spearman correlations of ratio and percent survival for different lipid concentrations ranging between − 0.82 and − 0.98), providing more evidence for the toxicity of the cationic lipid DOTAP, and the beneficial effect of chol on lipid toxicity (20). Similar results were obtained with respect to lipid concentration in both non-PEGylated (Spearman correlation = − 0.68) and PEGylated (− 0.59) LNPs, since higher lipid concentration caused greater toxicity. The effect of PEGylation was also clearly indicated in this figure. At every DOTAP/chol ratio, as well as at every lipid concentration, non-PEGylated LNPs resulted in lower number of viable SK-OV-3 cells compared to the PEGylated counterparts (statistically significant differences were seen for all comparisons at 125 µM, all but the lowest DOTAP/chol ratio at 62.5 µM, and for DOTAP/chol ratio of 1:1 at 31.25 µM), demonstrating that surface modification by adding PEG reduced the toxicity of LNPs (overall p < 0.0001).
Fig. 3.
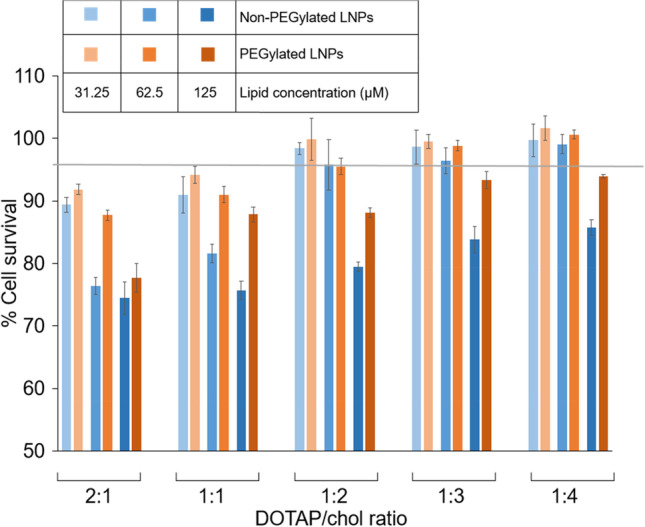
Cell viability assays. SK-OV-3 cells were treated with DOTAP/chol LNPs (blue bars) or PEGylated DOTAP/chol LNPs (orange bars) at five DOTAP/chol molar ratios (2:1, 1:1, 1:2, 1:3, and 1:4) and three lipid concentrations (31.25, 62.5, and 125 µM). After a 24-h incubation, MTS assays were performed to test the viability of the cells. Data are presented as mean ± SD (n = 4)
In Vitro mRNA Transfection of DOTAP/chol Lipoplexes
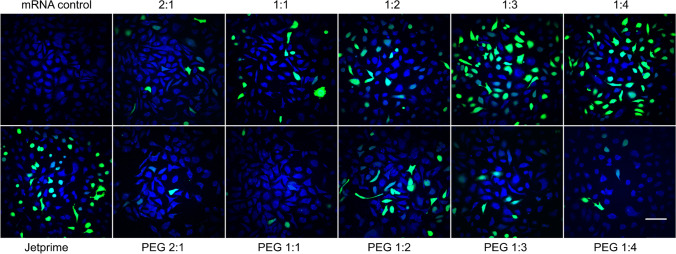
To determine the optimal ratio of PEGylated and non-PEGylated LNPs to mRNA for the best cellular transfection, we complexed different concentrations of lipid (31.25, 62.5, and 125 µM) with 1 μg of GFP-expressing mRNA and added them to the cells in full medium for 24 h. As shown in Fig. 4, the most efficient DOTAP/chol lipoplexes for mRNA transfection were at a 1:3 molar ratio and 62.5 µM lipid concentration without PEGylation (49.4 ± 2.12%). Compared to commercial mRNA transfection reagent JetPRIME (38.06 ± 3.68%), this formulation showed significantly higher transfection efficiency (p = 0.017). When the DOTAP/chol molar ratio was decreased from 1:3 to 1:4, or increased from 1:3 to 2:1, the mRNA transfection efficiency gradually decreased. A severe reduction in transfection efficiency due to PEGylation was observed (p < 0.0001). In addition, PEGylated lipoplexes had a different DOTAP/chol molar ratio–mediated trend in transfection efficiency compared to non-PEGylated ones; they achieved the highest efficiency at 1:2 DOTAP/chol ratio (19.3 ± 1.01%). PEGylated and non-PEGylated lipoplexes share a common feature related with the influence of lipid concentration. Their transfection efficiencies both increased from 31.25 to 62.5 µM, plateaued at 62.5 µM, and decreased from 62.5 to 125 µM, yet no evident discrepancy in transfection capacity was found between 31.25 and 125 µM. Therefore, 62.5 µM lipid concentration was used for all further stability and transfection experiments. SK-OV-3 cells treated with 62.5 µM non-PEGylated and PEGylated DOTAP/chol LNPs at five DOTAP/chol molar ratios (2:1, 1:1, 1:2, 1:3, and 1:4) complexed with a fixed amount of 1 μg mRNA by SRS and fluorescence microscopy are shown in Fig. 5. These images supported the trends discovered by flow cytometry that 1:3 DOTAP/chol ratio and 1:2 DOTAP/chol ratio resulted in the highest transfection efficiency in non-PEGylated lipoplexes and PEGylated lipoplexes, respectively.
Fig. 4.
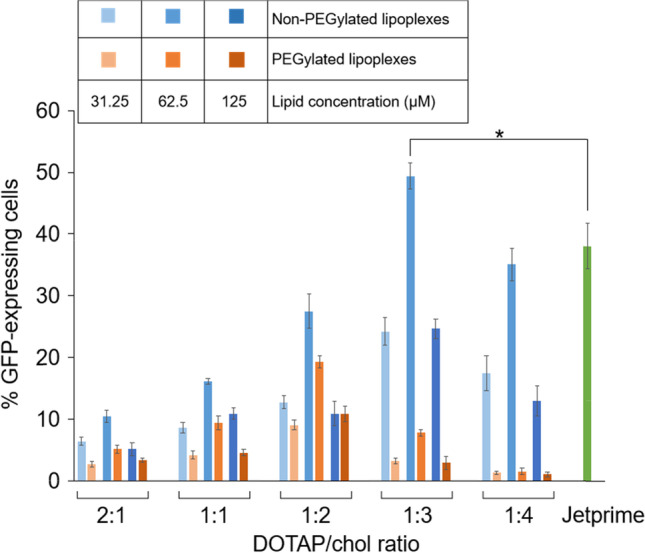
mRNA transfection assays. Non-PEGylated DOTAP/chol LNPs (blue bars) or PEGylated DOTAP/chol LNPs (orange bars) at five DOTAP/chol molar ratios (2:1, 1:1, 1:2, 1:3, and 1:4) and three lipid concentrations (31.25, 62.5, and 125 µM) were complexed with GFP-expressing mRNA (a fixed amount of 1 μg). The transfection efficiency was determined by the percentage of GFP-expressing cells against all cells counted using flow cytometry. Data are presented as mean ± SD (n = 3)
Fig. 5.
Stimulated Raman scattering (SRS) and fluorescence images of SK-OV-3 cells treated with 62.5 µM non-PEGylated and PEGylated DOTAP/chol LNPs at five DOTAP/chol molar ratios (2:1, 1:1, 1:2, 1:3, and 1:4) complexed with a fixed amount of 1 μg mRNA. Naked mRNA and JetPRIME were used to treat the cells as a negative control and a positive control, respectively. The images show overlap of the cell protein (blue) and GFP fluorescence (green) distribution in the cells. Scale bar: 100 µm
In Vitro pDNA and Oligonucleotide (Oligo) Transfection of DOTAP/chol Lipoplexes
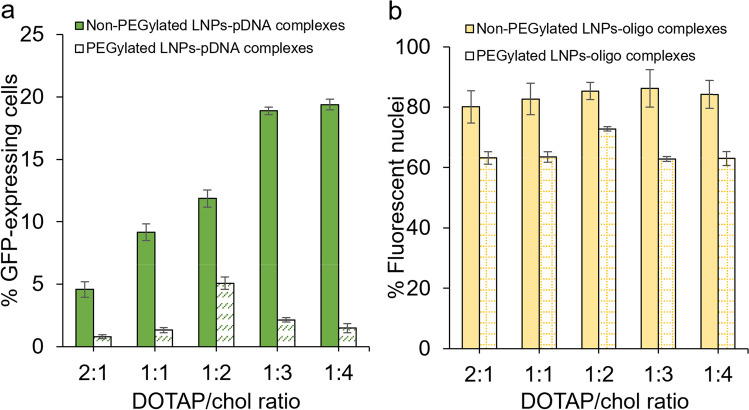
The ability of DOTAP/chol LNPs to deliver pDNA or oligo to the cell nucleus was investigated. In comparison to mRNA transfection, similar results were obtained regarding how DOTAP/chol molar ratio affects the pDNA transfection efficiency (Fig. 6a). For non-PEGylated lipoplexes, the transfection ability increased as the ratio decreased from 2:1 to 1:3, plateaued at 1:3, and was maintained without reduction at a 1:4 DOTAP/chol ratio. For PEGylated lipoplexes, transfection efficiency increased from 2:1 to 1:2, plateaued at 1:2, and decreased from 1:2 to 1:4. No obvious benefit existed in the delivery ability of non-PEGylated lipoplexes when changed (p = 0.61; Fig. 6b). Although the highest oligo transfection efficiency was tested in 1:2 DOTAP/chol lipoplexes, which is the same best ratio in mRNA and pDNA transfection studies, the other lipoplexes with different DOTAP/chol ratios showed very similar transfection efficiencies. Regarding the role of PEG modification on pDNA and oligo delivery, PEGylated LNPs showed decreased efficiency when transfecting pDNA (p < 0.0001; Fig. 6a) or oligo (p < 0.0001; Fig. 6b) compared to non-PEGylated ones, showing that PEGylation is detrimental not only to mRNA, but also pDNA and oligo transfection.
Fig. 6.
a pDNA transfection assays. Non-PEGylated or PEGylated DOTAP/chol lipoplexes containing 0.3 μg pDNA were added to SK-OV-3 cells and analyzed by flow cytometry after a 48-h treatment. b Oligonucleotide (oligo) transfection assays. SK-OV-3 cells were treated with non-PEGylated and PEGylated DOTAP/chol lipoplexes containing 1 μg FAM-labeled oligonucleotide. Data are presented as mean ± SD (n = 3)
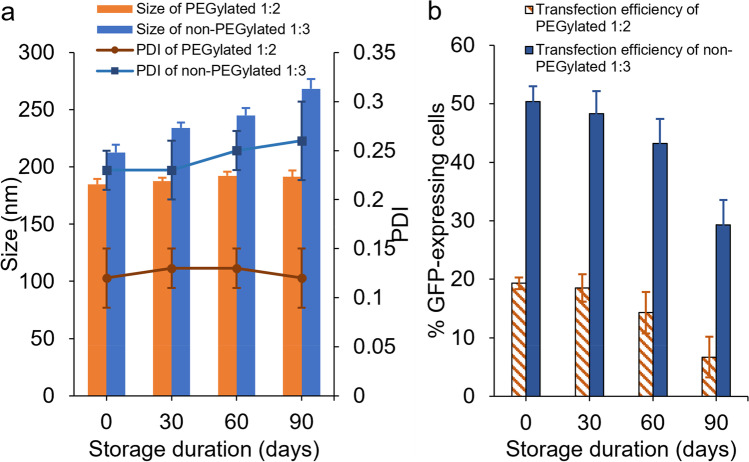
Stability of Non-PEGylated 1:3 and PEGylated 1:2 DOTAP/chol Lipoplexes
Based on previous results that 1:3 and 1:2 DOTAP/chol lipoplexes excelled at transfection efficiency among non-PEGylated and PEGylated lipoplexes, respectively, these two formulations were inspected for their stability. This experiment was carried out by testing the change in size, PDI, and mRNA transfection efficiency after 30, 60, and 90 days storage at 4 °C. As demonstrated in Fig. 7a, PEGylated 1:2 lipoplexes were able to maintain their size (p = 0.25) and PDI (p = 1.00) after 90 days of storage compared to the day 0 sample. Non-PEGylated 1:3 lipoplexes showed no significant difference (p = 0.55) in PDI over 90 days but exhibited significant difference (p = 0.01) in size within 30 days of storage (Fig. 7a). More importantly, the mRNA transfection efficiencies of both formulations were not significantly different (p = 0.12 for non-PEGylated 1:3, p = 0.14 for PEGylated 1:2), compared to their freshly prepared counterparts after 60 days of storage (Fig. 7b).
Fig. 7.
The stability of non-PEGylated 1:3 DOTAP/chol and PEGylated 1:2 DOTAP/chol lipoplexes. a The size and PDI changes of non-PEGylated 1:3 and PEGylated 1:2 DOTAP/chol lipoplexes with different storage time at 4 °C. b mRNA transfection efficiency in SK-OV-3 cells after transfection with fresh or stored 62.5 µM non-PEGylated 1:3 or PEGylated 1:2 DOTAP/chol lipoplexes containing the same amount of mRNA (1 μg). Data are shown as mean ± SD (n = 3)
DISCUSSION
DOTAP/chol LNPs have been classified as one of the most efficient vectors for gene transfection in in vivo experiments and clinical trials (5, 7, 28). However, there is still potential to improve aspects of the design of DOTAP/chol cationic LNPs. We previously showed that PEGylation, as well as cationic/neutral lipid ratio, plays a role in physical characterization, cytotoxicity, and DNA delivery of lipid-based delivery vehicles (16). In the current study, we prepared DOTAP/chol LNPs and expanded the influencing factors to include lipid to mRNA ratio to study their effects on mRNA transfection ability. Furthermore, the pDNA and oligonucleotide delivery ability of DOTAP/chol LNPs was also inspected. Since DNA and mRNA molecules require different intracellular transport routes, the aforementioned influence factors may generate different effects on DNA and mRNA transfection.
We observed that physicochemical properties such as the size and PDI of the DOTAP/chol LNPs were not affected by varying DOTAP/chol molar ratios; only the surface charge was shown to reduce as the amount of cationic lipid DOTAP decreased in the formulation. The increase in size after complexation with mRNA indicated the good interaction between cationic vesicles and nucleic acids. The average diameters of lipoplexes were shown to be around 200 nm, which is a size described to be suitable for gene delivery (29). We also showed that PEGylated LNPs resulted in reduced size and ζ-potential when complexed with DNA or RNA compared to non-PEGylated ones, which is in accordance with previous work (16, 24). The incorporation of negatively charged DSPE-PEG not only led to a decrease in surface charge of LNPs, but also decreased size by enhancing the repulsive forces on the nanoparticle surface, causing condensing of the inner hydrophilic cavity in the vesicles (30, 31).
We then investigated how DOTAP/chol ratio, lipid concentration, and PEGylation affect cell viability. In nucleic acid delivery, the presence of positive-charged lipids is beneficial to achieve sufficient electrostatic interaction with nucleic acids and cell membrane. Since cationic lipids are not natural constituents of cells, their biocompatibilities should be considered. Our results showed that higher DOTAP/chol molar ratio and/or the lipid concentration caused greater decrease of viable cells. This is in agreement with the literature reporting the dose-dependent cytotoxicity of cationic lipids such as DOTAP (21, 26). Compared to non-PEGylated DOTAP/chol LNPs, PEGylation resulted in improved viability. It has been illustrated that surface charge is positively correlated with the toxicity of nanoparticles (32). Hence, the charge shielding effect of PEGylation on the surface charge of cationic groups might lead to lessened toxicity. Overall, we have demonstrated that the concentration of LNPs, as well as the quantity of cationic lipid DOTAP, affects the biocompatibility of LNPs in a negative manner, while the presence of PEG molecule shows beneficial impact on LNP toxicity.
Despite its toxicity at high concentrations, DOTAP is still a good choice of cationic lipid because the biodegradable ester linker is considered to be less toxic than lipids with an ether linker, such as (N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) (33, 34). In terms of neutral or helper lipids, dioleoyl phosphatidylethanolamine (DOPE) and chol are the most commonly used. Compared to chol, DOPE excels in membrane destabilization and thus allows for the efficient escape of LNPs from the endosomal vesicles (35). However, when used under serum conditions, DOPE-containing LNPs show a less satisfactory nucleic acid delivery effect due to the lack of adequate fusion with the cell membrane (36). Chol, on the other hand, can undermine the interaction between serum proteins and lipoplexes, as well as form separated nanodomains on the surface of lipid vesicles, contributing to the stability and outstanding efficiency of LNPs in serum-containing media (9, 37). Therefore, chol is a better option for in vivo transfection or transfection under physiological conditions.
The molar ratio of DOTAP/chol is one of the key parameters determining transfection efficiency. Using GFP-expressing mRNA, we found that the 1:3 DOTAP/chol LNPs exhibited a significantly higher percent of GFP-expressing cells than all the formulations tested including a commercial mRNA transfection reagent. This ratio also worked well for pDNA transfection with approximately the same highest transfection efficiency as 1:4 DOTAP/chol. Even though PEGylation resulted in decreased mRNA, pDNA, and oligo delivery efficiency, it is still important to probe into the mechanism of PEGylated LNPs since PEG modification is beneficial for prolonged systemic circulation (22). The optimal DOTAP/chol ratio for PEGylated formulations, 1:2, remains to be constant for mRNA, pDNA, and oligo transfection. We also assessed the effect of lipid/mRNA ratio on transfection. For both PEGylated and non-PEGylated lipoplexes, the mRNA transfection efficiency increased and then decreased as the lipid concentration increased, in line with a former study (26). We could hypothesize that lower lipid concentration induced less lipoplex-cell interaction as well as endosomal escape due to low surface charge. But when lipid concentration is too high, it is difficult for mRNA to dissociate from lipoplex to function in the cells (20). Another possible explanation is that the cytotoxicity of high lipid concentration interfered with transfection ability (24).
In order to move this biotherapeutic delivery approach forward for future clinical use, therapeutic stability is an important aspect to be investigated. For instance, the instability of vaccines often poses challenges during clinical development from laboratory to clinic and commercial distribution from factory to patient (38). Our data indicate that our lipoplexes can be safely stored at 4 °C for at least 60 days and do not result in a reduction in transfection efficiency. This excellent stability can be attributed to the surface charge of these LNPs that provided enough repulsive force for these particles to avoid aggregation, as well as the function of chol to modulate the release of hydrophilic molecules such as mRNA from lipid vesicles (39).
CONCLUSION
In conclusion, the present study shows that the addition of PEG in DOTAP/chol LNPs aids in nanoparticle size distribution and biocompatibility but decreases nucleic acid binding affinity and transfection efficiency. In addition, the cytotoxicity of these LNPs is positively correlated with the percentage of the cationic lipid DOTAP in the formulation as well as the overall lipid concentration. More importantly, we are able to determine the optimal formulations for mRNA transfection in PEGylated and non-PEGylated lipoplexes with a specific lipid to mRNA ratio and DOTAP to chol ratio. These two formulations are non-invasive and have been tested to remain stable for 60 days at 4 °C. Additionally, the non-PEGylated one is more efficient compared to a commercially available mRNA transfection reagent. Other than successful mRNA transfection, DOTAP/chol LNPs have demonstrated the capability of transfecting pDNA and oligonucleotide to the cell nuclei. This method of optimization and formulation screening will lead to the design of better adapted DOTAP/chol LNPs for a wide range of gene therapy applications.
Acknowledgements
The authors thank Mohammad Ali and Deanna Maybee of the Ali Laboratory (Binghamton University) for their plasmid DNA, Effectene, and helpful discussions pertaining to this work. We also thank the Binghamton University School of Pharmacy for funding and support.
Author Contribution
Mengwei Sun: investigation, methodology, writing-original draft, writing-review and editing.
Utkarsh J Dang: data analysis.
Yuhao Yuan: methodology, investigation.
Alexandra Maria Psaras: methodology, investigation.
Ositomiwa O Osipitan: writing-review and editing.
Tracy A Brooks: supervision, writing-review and editing.
Fake Lu: supervision, writing-review and editing.
Anthony J Di Pasqua: conceptualization, supervision, methodology, writing-review and editing.
Funding
This research was partially supported by the National Institutes of Health under award number R15GM140444 (Frank Lu).
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 3.Gjetting T, Arildsen NS, Christensen CL, Poulsen TT, Roth JA, Handlos VN, et al. In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection. Int J Nanomed. 2010;5:371–383. doi: 10.2147/ijn.s10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawano T, Okuda T, Aoyagi H, Niidome T. Long circulation of intravenously administered plasmid DNA delivered with dendritic poly(L-lysine) in the blood flow. Journal of controlled release : official journal of the Controlled Release Society. 2004;99(2):329–337. doi: 10.1016/j.jconrel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y, et al. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7(1):11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S-Y, Lee S-J, Lim S-J. Formulation and in vitro and in vivo evaluation of a cationic emulsion as a vehicle for improving adenoviral gene transfer. Int J Pharm. 2014;475(1):49–59. doi: 10.1016/j.ijpharm.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Vupputuri S, Tayebi L, HikkaduwaKoralege RS, Nigatu A, Mozafari M, Mishra A, et al. Polyethylene glycol–modified DOTAP:cholesterol/adenovirus hybrid vectors have improved transduction efficiency and reduced immunogenicity. J Nanopart Res. 2021;23(2):37. doi: 10.1007/s11051-020-05134-9. [DOI] [Google Scholar]
- 8.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochimica et Biophysica Acta (BBA) Biomembranes. 2008;1778(10):2177–81. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Köster F, Finas D, Schulz C, Hauser C, Diedrich K, Felberbaum R. Additive effect of steroids and cholesterol on the liposomal transfection of the breast cancer cell line T-47D. Int J Mol Med. 2004;14(4):769–772. [PubMed] [Google Scholar]
- 10.Templeton NS. Nonviral delivery for genomic therapy of cancer. World J Surg. 2009;33(4):685–697. doi: 10.1007/s00268-008-9825-0. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Dong X, Luo Y, Zhang G, Shan J, Wang Q, et al. Construction of human MASP-2-CCP1/2SP, CCP2SP, SP plasmid DNA nanolipoplexes and the effects on tuberculosis in BCG-infected mice. Microb Pathog. 2017;109:200–208. doi: 10.1016/j.micpath.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Li J, Liu Q, Song W, Zhang X, Tiruthani K, et al. Local blockade of interleukin 10 and C-X-C motif chemokine ligand 12 with nano-delivery promotes antitumor response in murine cancers. ACS Nano. 2018;12(10):9830–9841. doi: 10.1021/acsnano.8b00967. [DOI] [PubMed] [Google Scholar]
- 13.Sicard G, Paris C, Giacometti S, Rodallec A, Ciccolini J, Rocchi P, et al. Enhanced antisense oligonucleotide delivery using cationic liposomes grafted with trastuzumab: a proof-of-concept study in prostate cancer. Pharmaceutics. 2020;12(12):1166. [DOI] [PMC free article] [PubMed]
- 14.Brégeon D, Doetsch PW. Transcriptional mutagenesis: causes and involvement in tumour development. Nat Rev Cancer. 2011;11(3):218–227. doi: 10.1038/nrc3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. [DOI] [PMC free article] [PubMed]
- 16.Sun M, Yuan Y, Lu F, Di Pasqua AJ. Physicochemical factors that influence the biocompatibility of cationic liposomes and their ability to deliver DNA to the nuclei of ovarian cancer SK-OV-3 cells. Materials (Basel). 2021;14(2):416. [DOI] [PMC free article] [PubMed]
- 17.Yang SY, Zheng Y, Chen JY, Zhang QY, Zhao D, Han DE, et al. Comprehensive study of cationic liposomes composed of DC-Chol and cholesterol with different mole ratios for gene transfection. Colloids Surf, B. 2013;101:6–13. doi: 10.1016/j.colsurfb.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Sun J, Li A, Chen D. The promoted delivery of RRM2 siRNA to vascular smooth muscle cells through liposome-polycation-DNA complex conjugated with cell penetrating peptides. Biomed Pharmacother. 2018;103:982–988. doi: 10.1016/j.biopha.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 19.Roberts R, Al-Jamal WT, Whelband M, Thomas P, Jefferson M, van den Bossche J, et al. Autophagy and formation of tubulovesicular autophagosomes provide a barrier against nonviral gene delivery. Autophagy. 2013;9(5):667–682. doi: 10.4161/auto.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao J, Ding WF, Che XH, Chen YC, Chen F, Chen XD, et al. Optimization of a cationic liposome-based gene delivery system for the application of miR-145 in anticancer therapeutics. Int J Mol Med. 2016;37(5):1345–1354. doi: 10.3892/ijmm.2016.2530. [DOI] [PubMed] [Google Scholar]
- 21.Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials (Basel). 2020;10(2):190. [DOI] [PMC free article] [PubMed]
- 22.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini ES, Nikkhah M, Hosseinkhani S. Cholesterol-rich lipid-mediated nanoparticles boost of transfection efficiency, utilized for gene editing by CRISPR-Cas9. Int J Nanomed. 2019;14:4353–4366. doi: 10.2147/IJN.S199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Li H, Sun J, Gao J, Liu W, Li B, et al. DC-Chol/DOPE cationic liposomes: a comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. Int J Pharm. 2010;390(2):198–207. doi: 10.1016/j.ijpharm.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Brgles M, Šantak M, Halassy B, Forcic D, Tomašić J. Influence of charge ratio of liposome/DNA complexes on their size after extrusion and transfection efficiency. Int J Nanomed. 2012;7:393–401. doi: 10.2147/IJN.S27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsana H, Olusanya TOB, Carr-wilkinson J, Darby S, Faheem A, Elkordy AA. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci Rep. 2019;9(1):15120. doi: 10.1038/s41598-019-51065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psaras AM, Chang KT, Hao T, Brooks TA. Targeted downregulation of MYC through G-quadruplex stabilization by DNAi. Molecules. 2021;26(18):5542. [DOI] [PMC free article] [PubMed]
- 28.Lu C, Stewart DJ, Lee JJ, Ji L, Ramesh R, Jayachandran G, et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE. 2012;7(4):e34833. doi: 10.1371/journal.pone.0034833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Junquera E, Aicart E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv Coll Interface Sci. 2016;233:161–175. doi: 10.1016/j.cis.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Garbuzenko O, Barenholz Y, Priev A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem Phys Lipids. 2005;135(2):117–129. doi: 10.1016/j.chemphyslip.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Tang J, Kuai R, Yuan W, Drake L, Moon JJ, Schwendeman A. Effect of size and pegylation of liposomes and peptide-based synthetic lipoproteins on tumor targeting. Nanomedicine. 2017;13(6):1869–1878. doi: 10.1016/j.nano.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269(4):2550–2561. doi: 10.1016/S0021-9258(17)41980-6. [DOI] [PubMed] [Google Scholar]
- 34.Belmadi N, Midoux P, Loyer P, Passirani C, Pichon C, Le Gall T, et al. Synthetic vectors for gene delivery: an overview of their evolution depending on routes of administration. Biotechnol J. 2015;10(9):1370–1389. doi: 10.1002/biot.201400841. [DOI] [PubMed] [Google Scholar]
- 35.Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, et al. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11(5):801–810. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Kim SY, Lee SJ, Kim JK, Choi HG, Lim SJ. Optimization and physicochemical characterization of a cationic lipid-phosphatidylcholine mixed emulsion formulated as a highly efficient vehicle that facilitates adenoviral gene transfer. Int J Nanomed. 2017;12:7323–7335. doi: 10.2147/IJN.S146785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, Anchordoquy TJ. Cholesterol domains in cationic lipid/DNA complexes improve transfection. Biochem Biophys Acta. 2008;1778(10):2177–2181. doi: 10.1016/j.bbamem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–259. doi: 10.1016/j.biologicals.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Kaddah S, Khreich N, Kaddah F, Charcosset C, Greige-Gerges H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2018;113:40–48. doi: 10.1016/j.fct.2018.01.017. [DOI] [PubMed] [Google Scholar]