| A1
|
n-BuLi, ether, CF3COOEt, −78 °C, 2 h |
49,53
|
|
n-BuLi, THF, CF3COOMe, −78 °C, 2 h |
54
|
| Mg, CF3COR, R = piperidinyl |
55
|
| Mg/CF3COOH or n-BuLi/CF3COR, R = OMe, piperidinyl |
20
|
| A2
|
(1) TMS-CF3, catalytic TBAF, THF; then aqueous HCl |
56
|
| (2) Dess-Martin periodinane, TFA, CH2Cl2
|
| (1) TMS-CF3, K2CO3, DMA, rt, 2 h |
57
|
| (2) 1 M HCl, rt, 1 h |
| (3) Dess-Martin, CH2Cl2, rt, 8 h |
| B1
|
NH2OH, NaOH, EtOH, reflux, 21 h |
49
|
| NH2OH·HCl, pyridine, EtOH, 60 °C, 4 h |
53–58
|
| NH2OH·HCl, NaOH, EtOH, reflux, 16 h or pyridine, 70 °C, 3 h |
59
|
| B2
|
1 M LiN(TMS)2 in THF, toluene, 0 °C |
51
|
| C |
MsCl, TEA |
49
|
| TsCl, pyridine, reflux |
2,53,54,56,59
|
| TsCl, TEA, N,N-dimethylaminopyridine, CH2Cl2, 0 °C, 45 min |
55
|
| TsCl, DMAP, TEA, CH2Cl2, rt, 45 min |
57
|
| D1
|
NH3 (l), CH2Cl2, −78 °C |
49,54,55
|
| NH3 (l), ether, −78 °C→rt or rt |
53,56,57,59
|
| NH3 (l), 80 °C, 20 h |
58
|
| D2
|
NH3 (l), LiNH2, rt, 11 h |
50,57,59
|
| M |
MeOH, 18 h |
51
|
| N |
(1) 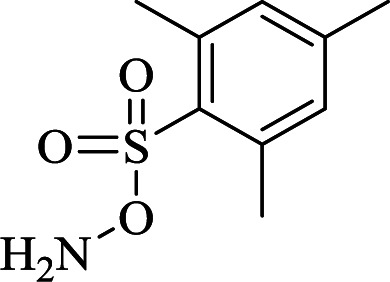 in THF, 0 °C in THF, 0 °C |
51
|
| (2) piperidine |
| E |
Ag2O, ether, 23 °C, 3.5 h |
49,54
|
| tert-BuOCl, TEA |
2,55
|
| I2, TEA, CH2Cl2 or MeOH, 0 °C |
56,58,59
|
| MnO2, ether or CH2Cl2, rt |
53,57
|
| (COCl)2, DMSO, DCM, −78 °C |
60
|
 in THF, 0 °C
in THF, 0 °C