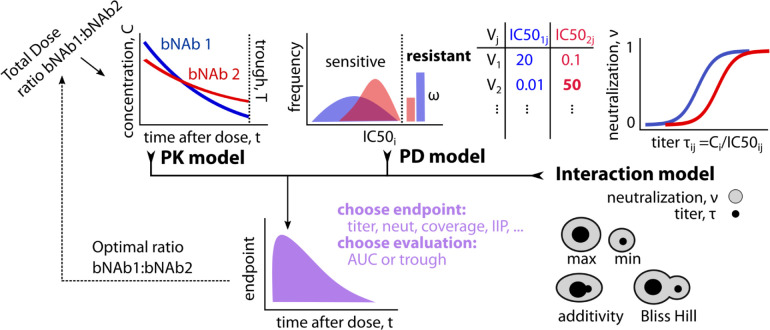
Fig 1. PKPD model schematic for optimizing combination bNAb treatment against a genetically diverse pathogen like HIV.
The model incorporates: pharmacokinetics (PK), pharmacodynamics (PD), and interactions between broadly neutralizing antibodies (bNAbs). PK quantifies bNAb concentrations over time after administration. PD quantifies potencies at a given concentration for each antibody against many viral strains with sensitivity determined by IC50ij, the level of the i-th drug needed to achieve 50% neutralization of the j-th viral strain–with some fraction ω of strains completely resistant. Titer, or the ratio of concentration to IC50 of each antibody against a certain strain, maps to neutralization proportion (0–1 scale) of viral infection events that are blocked. Interaction model includes taking either the worst (minimum) or the best (maximum) titer/neutralization between two products. Two more mechanistic interaction models combine titers (additivity) or neutralization (Bliss Hill), and generally mean combinations outperform the best single bNAb. Depending on the PKPD outcome measure of interest and when that measure is evaluated (throughout the study = AUC, at the low point = trough), we identify the optimal ratio of bNAbs.