Abstract
Objectives:
Sepsis is a significant cause of morbidity and mortality. Children with sepsis often have alterations in microcirculation and vascular permeability. Our objective is current evidence regarding the role of the endothelial glycocalyx as a determinant of capillary leakage in these patients.
Data Sources:
We reviewed PubMed, EMBASE, and Google scholar using MeSH terms “glycocalyx”, “fluids”, “syndecan”, “endothelium”, “vascular permeability”, “edema”, “sepsis”, “septic shock”, “children”.
Study Selection:
Articles in all languages were included. We include all studies in animals and humans related to glycocalyx and vascular permeability.
Data Extraction:
Studies in children and adults, as well as animal studies, were included.
Data Synthesis:
One of the fundamental components of the endothelial barrier structure is the glycocalyx. It is a variable thickness layer distributed throughout the whole body, which fulfills a very important function for life: the regulation of blood vessel permeability to water and solutes, favoring vascular protection, modulation, and hemostasis. In the last few years, there has been a special interest in glycocalyx disorders and their relationship to increased vascular permeability, especially in patients with sepsis in whom the alterations that occur in the glycocalyx are unknown when they are subjected to different water resuscitation strategies, vasopressors, etc. This review describes the structural and functional characteristics of the glycocalyx, alterations in patients with sepsis, with regard to its importance in vascular permeability conservation and the possible impact of strategies to prevent and/or treat the injury of this fundamental structure.
Conclusions:
The endothelial glycocalyx is a fundamental component of the endothelium and an important determinant of the mechanotransduction and vascular permeability in patients with sepsis. Studies are needed to evaluate the role of the different types of solutions used in fluid bolus, vasoactive support, and other interventions described in pediatric sepsis on microcirculation, particularly on endothelial integrity and the glycocalyx.
Keywords: edema, endothelium, fluids, septic shock, vascular tone
Sepsis is characterized by an inadequate and progressive response of the organism to an infection. It is one of the main health problems affecting human beings all over the world, with costs exceeding 20 billion dollars per year in the United States, which is equivalent, on average, to 5.2% of all hospitalization costs in that country (1). In spite of multiple efforts using evidence-based medicine strategies, the average mortality in children continues to be disturbingly high in developed (19.3%) and developing (31.7%) countries (2, 3).
A fundamental characteristic of the pathophysiologic process associated with sepsis is the individual’s abnormal immunologic response, where an imbalance in the production of pro- and anti-inflammatory cytokines is typically seen, leading to significant multisystemic involvement (4). One of the organs which is usually affected by this involvement in the initial phases of the disease, in particular by the prothrombotic, inflammatory, and fibrinolytic phenomena associated with sepsis, is the vascular endothelium. Injury to this organ leads to increased vascular permeability, vasodilation, and extravasation of fluid from the intravascular to the interstitial space, producing a state of relative hypovolemia which affects tissue perfusion pressure and oxygen delivery to the tissues (5, 6).
The structure responsible for helping to control and avoid the exit of this fluid is the endothelial glycocalyx (4, 5). This layer of proteoglycans and glycosaminoglycans, located on the intraluminal face of the blood vessels and covering the endothelial cells, plays a determining role in numerous physiologic processes including inflammation, microvascular permeability, and endothelial mechanotransduction (i.e. detection and transduction of mechanical forces) (6-8). However, the precise glycocalyx disruptions in the septic patient, along with their triggers, recovery, and the possibility of damage secondary to therapeutic interventions (such as balanced and unbalanced crystalloid boluses, vasoactive medications, etc.), are unknown. Recognizing the glycocalyx’s role in the pathophysiology of this disease will allow us to understand it better and proposes a therapeutic approach to sepsis beginning with the microcirculation rather than the macrocirculation, as is currently done. The goal of this article is to review the basic science of the glycocalyx and its alteration related to vascular permeability in patients with sepsis.
SEARCH STRATEGY AND ARTICLE SELECTION
A systematic literature search in English was performed using the PubMed, EMBASE and Google scholar using MeSH terms “glycocalyx”, “fluids”, “syndecan”, “endothelium”, “vascular permeability”, “edema”, “sepsis”, “septic shock”, and “children” between January 1990 and January 2019. A total of 235 articles were found, of which the most relevant ones were selected: articles which discussed the basic science of the glycocalyx and the clinical aspects related to vascular permeability, sepsis, biomarkers and therapeutic strategies aimed at preventing and repairing damage.
Structure of the Glycocalyx: Protective Barrier
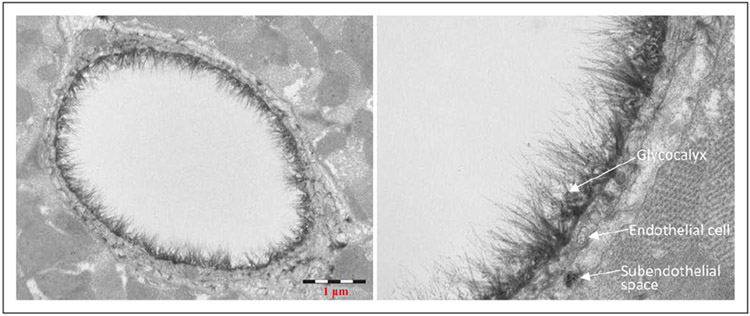
The glycocalyx is a fine layer which covers the interior of human blood vessels, from the capillaries to the arteries and veins, forming an interface between the endothelial cells and blood stream (7, 9). It was initially described by Luft in 1966 when it was observed in its totality using electron microscopy and special stains such as ruthenium red and alcian blue, which permitted the visualization of carbohydrates. With the first stain it is seen as an amorphous or grainy material, and with the second as a net of fine filaments (Fig. 1) (10).
Figure 1.
Electron micrograph of a goat coronary capillary stained with alcian blue. Courtesy of B.M. van den Berg, Leiden University Medical Center, The Netherlands.
The glycocalyx constitutes a structural portion of the endothelium which contributes substantially toward maintaining its integrity (11). Its thickness may vary, depending on its location and the blood vessels studied, but tends to range from 0.1 to 1 micron. It has been isolated in endothelial cell cultures beginning the first week of culture, both under physiologic as well as pathologic conditions, such as those seen in septic patients. Animal and human models have shown that, under inflammatory conditions, the glycocalyx takes 5–7 days to begin its recovery, after which it can reestablish its endothelial and microcirculatory functions (12-14).
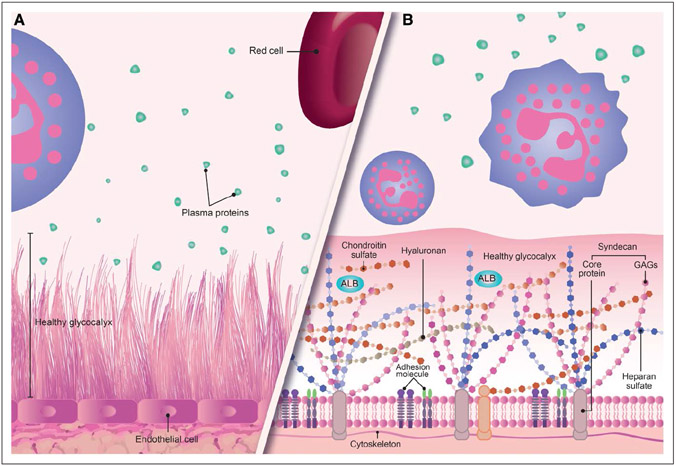
The glycocalyx is composed of proteoglycans, glycosaminoglycans, and glycoproteins, as the nonsoluble, endothelial cell-dependent components (Fig. 2) (13). Of the first component, 50–90% are heparan sulfate proteoglycans (e.g., syndecans, glypicans, perlecans) which are anchored to the endothelial cell through the transmembrane domain of its protein core (4, 9). Proteoglycans are considered to be the main axis of the glycocalyx, to which the glycosaminoglycan chains are joined, leading to the production of soluble proteoglycans, which are located in the upper portion of this structure. The glycosaminoglycans which are joined to proteoglycans include heparan sulfate, chondroitin sulfates, dermatan sulfates, and keratin sulfates. The sulfate and the glycosaminoglycans have given them a negative charge, enabling electrostatic interaction with plasma proteins. Hyaluronan (hyaluronic acid) is another glycosaminoglycan which is not sulfated and does not have a negative charge (it is not joined to the transmembrane protein) but interacts with other glycosaminoglycans to retain water and stabilize the gel-like structure of the glycocalyx. As shown in Figures 2 and 3, proteins such as albumin, fibrinogen, thrombomodulin, antithrombin III, and fibronectin, as well as cell adhesion molecules, can interact with the glycosaminoglycans, and this interaction may be responsible for many of the functional properties of these proteins. Hyaluronan is synthesized and degraded at a rate of 5 grams/d (14-16).
Figure 2.
An approximate graphic representation of the glycocalyx. A, A graphic representation of the healthy glycocalyx and its relation to vascular structures and plasma proteins. B, An enlargement of the image. The proteoglycans are shown, principally syndecan with its transmembrane protein core joined to the glycosaminoglycans (GAGs) and interacting with proteins such as albumin (ALB-green). The other glycocalyx component is also shown as the glycoproteins (adhesion molecule).
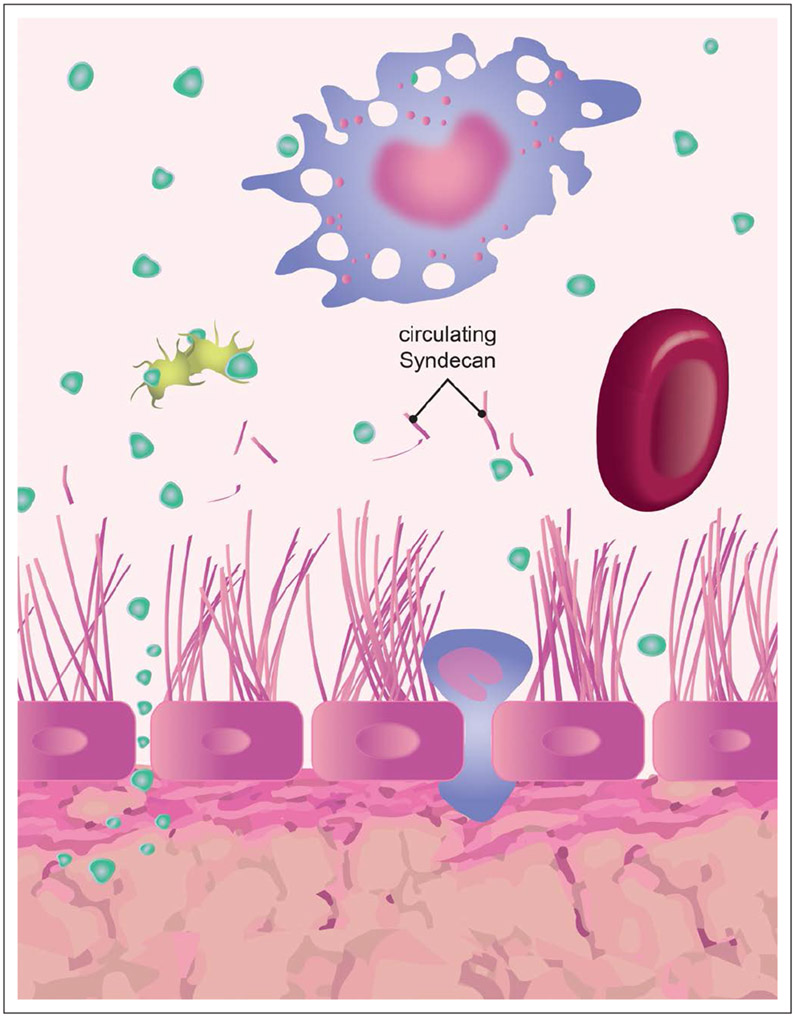
Figure 3.
Glycocalyx damage and increased permeability in sepsis. The figure depicts the passage of molecules and fluids through the damaged glycocalyx as well as the release of its degradation products (syndecans) into systemic circulation. A platelet, RBC, and macrophage are shown interacting with plasma proteins (green points) and the glycocalyx. Green points represent the transendothelial migration of proteins when the glycocalyx is injured.
The glycoprotein component is dominated by cell adhesion molecules (e.g. E-selectin, P-selectin, integrins), as well as protein complexes relevant to hemostasis (glycoprotein Ib-IX-V complex, which binds to the von Willebrand factor and P-selectin), mediating the interaction of the platelets with the activated endothelial cells (17). Microenvironment disruptions such as pH modification or the elevated temperature which occurs in septic patients may affect these fundamental components of the glycocalyx and magnify the severity of the disease.
In addition, the glycocalyx has a soluble component which interacts with those anchored to the endothelial cell in order to give good stability, being in dynamic equilibrium with the blood flow, and dependent on special microenvironmental conditions such as pH and the production of membrane-bound elements by the Golgi bodies and endoplasmic reticulum of the endothelial cell (14). The soluble component of the glycocalyx is made up of a broad range of molecules, such as proteins and proteoglycans, which are produced by the endothelium or come from the systemic circulation. They may bind to the component joined to the endothelial cell through receptors or enzymes (fibroblast growth factor receptors, lipase lipoproteins, and low-density lipoproteins) or also by binding to the glycosaminoglycans, especially heparan sulfate, which has numerous protein binding sites due to its specific patterns of sulfation, as was previously explained (18, 19).
The Glycocalyx’s Role in Vascular Permeability
From a functional perspective, the glycocalyx acts as a protective layer of the endothelial cell; it also regulates the permeability of various molecules into and out of the endothelial cell, as well as the processes associated with glomerular filtration. It is a fundamental element in vascular mechanotransduction (i.e. the detection and transduction of mechanical forces) and also acts in hematocrit regulation (i.e. blood flow toward the capillaries), processes of cellular adhesion to the endothelium (i.e. of platelets and leukocytes), and hemostasis (20-22). Accordingly, the glycocalyx is thought to contribute to leukocyte migration through endothelial cells in patients with sepsis, which has been proven in pulmonary sepsis models. Lipopolysaccharide may be a useful tool for triggering glycocalyx degradation. This damage exteriorizes intercellular adhesion molecule (ICAM)–1 and/or vascular cell adhesion molecule 1 (VCAM-1) in the blood vessel lumen, which facilitates the interaction of leukocytes with the endothelium. The rolling leukocytes on the vessel wall may be seen in sepsis models where the leukocytes are labeled with rhodamine 6G, which helps to understand the importance of the glycocalyx in the various phases of leukocyte transmigration from the blood vessels to the site of infection (23-25) (Table 1).
TABLE 1.
Main Articles Regarding Glycocalyx Injury in Sepsis and Increased Vascular
| References | Type of Study |
Study Population |
Year | Sample Size |
Objectives | Glycocalyx Measurement |
Conclusions |
|---|---|---|---|---|---|---|---|
| Rehm et al (5) | Animal | Guinea pigs: weight 200–250 g | 2004 | 17 | Evaluate the impact on the endothelial glycocalyx for the extravasation of colloidal infusion solutions. | Electron microscopy | The endothelial glycocalyx acts as a competent barrier for water and colloids. |
| Alfieri et al (26) | Animal | Male mice (7–10 wk) | 2012 | 24 | Investigate the acute effects of an angiopoietin-1 derivative on microcirculation in a sepsis model, and its association with the inflammatory response. | Intravital microsopy | There is macrocirculation protection in sepsis models following administration of matrilin-1-angiopoietin-1 in endotoxemia-induced vascular dysfunction models through a reduction in inflammation, but without proangiogenic actions. |
| Mochizuki et al (19) | Animal | Adult dogs (weighing 10–32 kg) | 2003 | 31 | Investigate the role of the glycocalyx as a sensor and mechanical trigger of nitric oxide production in response to endothelial shear-stress forces. | Nitrite concentration | Hyaluronic acid glycosaminoglycans within the glycocalyx play a pivotal role in detecting and amplifying the shear force of flowing blood that triggers endothelium-derived nitric oxide. |
| Van Haaren et al (22) | Animal | Male rats (weigh 200–250 g) | 2003 | 24 | Determine the solute transport and endothelial permeability properties of the glycocalyx. | Electron microscopy | Demonstrated the presence of a permeability barrier to solutes on the luminal side of the endothelium in isolated small arteries. There is glycocalyx damage during oxidative stress. |
| Schmidt et al (25) | Animal | Mice | 2012 | 43 | Glycocalyx damage is associated with inflammation and capillary leakage in a sepsis model. | Intravital microscopy | Endothelial glycocalyx breakdown increases the expression of endothelial surface adhesion molecules and favors neutrophil adhesion as well as capillary leakage. |
| Steppan et al (27) | Human | Adults | 2011 | 150 | Evaluate glycocalyx damage in patients with sepsis compared with healthy volunteers and patients following major abdominal surgery. | Inflammatory and glycocalyx injury biomarkers: intercellular adhesion molecule-1, vascular cell adhesion molecule-1, interleukin-6, syndecan-1, heparan sulfate. | Elevated glycocalyx damage biomarkers were found in patients with sepsis and following major abdominal surgery, which could explain the capillary leak syndrome. |
| Rovas et al (28) | Human | Adults | 2019 | 40 | Investigate the associations between glycocalyx dimensions and established parameters of microcirculation dysfunction in sepsis. | Videomicroscopy; sublingual and atomic force microscopy | Glycocalyx damage can occur independently of microcirculatory impairment as measured by classical consensus parameters. Further studies in critically ill patients are needed to unravel the relationship of glycocalyx damage and microvascular impairment, as well as their prognostic and therapeutic importance in sepsis. |
| Kataoka et al (29) | Animal | BALB/c mice (Japan SLC, Inc, Shizuoka, Japan) (8–10 wk weighing 24–28 g) | 2017 | 26 | Evaluate the endothelial glycocalyx in an in vivo sepsis model. | Electron microscopy and intravital microscopy, syndecan-1 | Severe sepsis-induced morphologic degradation of the glycocalyx, accompanied by shedding of the syndecan-1 core protein and an increase in leukocyte–endothelial interactions affecting vascular permeability. |
| Hippensteel et al (30) | Human | Adults | 2019 | 100 | Glycocalyx degradation is associated with the volume of IV fluids administered during early sepsis resuscitation. | Heparan sulfate, syndecan-1 | Glycocalyx degradation occurs in sepsis and septic shock and is associated with inhospital mortality. The volume of IV fluids administered during sepsis resuscitation is independently associated with the degree of glycocalyx degradation. |
One of its most important functions is regulation of permeability to water and solutes (20, 21). Using special enzyme and dextran application techniques to degrade the glycocalyx, Van Haaren et al (22) showed that this structure is responsible for changes in water permeability caused by tension forces, and, when intact, is responsible for protecting against edema and protein filtration. Likewise, Henrich et al (8) have demonstrated that some proinflammatory cytokines (such as tumor necrosis factor alpha, which is elevated in patients with sepsis) may injure the glycocalyx, thus increasing vascular permeability and affecting tissue perfusion.
By the same token, the glycocalyx is known to play an important role in the mechanotransduction of forces applied to endothelial cells due to blood flow, especially stress and shear forces, which are fundamental determinants of endothelial morphology and functionality (15, 31-34). It appears that all of the glycocalyx’s components carry out this function together, as a whole. However, heparan sulfate and hyaluronic acid play a fundamental role in the detection and amplification of shear forces caused by blood flow, as shown by Mochizuki et al (19). This author and his group demonstrated, in a canine model, that the release of nitric oxide from the endothelial cell is regulated in part by the endothelial glycocalyx, conditioning a change in its structure related to shear-stress forces. This indicates that the glycocalyx has vasculoprotective effects which are mediated by the release of nitric oxide from the endothelial cell, facilitated by shear-stress forces, and the inhibition of leukocyte adhesion as well as by the activation of coagulation under various physiologic and pathologic circumstances, such as may occur in patients with sepsis (24, 26, 35, 36). Under inflammatory conditions, the activation and/or externalization of proteases or glycosidases can lead to the degradation of the glycocalyx through the digestion of proteoglycans and/or glycosaminoglycans. Loss of the endothelial glycocalyx, or glycocalyx shedding, may facilitate ligand-receptor interactions that promote the adhesion of leukocytes (7).
Likewise, the glycocalyx also regulates the passage of macromolecules, such as high-density lipoproteins and albumin, toward the extravascular space (12, 35, 35). The most superficial portion of the glycocalyx has a negative charge, related to the glycosaminoglycans (some acidic mucopolysaccharides) which contain a large quantity of carboxyl groups and sulfates. Many of the proteins found in plasma, such as albumin, also have a negative charge, which causes the glycocalyx layer to generate electrostatic repulsion to them, thus impeding their passage into the interstitial space. This situation is influenced in part by the thickness of the glycocalyx, which changes according to the blood vessel region in which it is found (25). Recently, Ueda et al (24) found that the passage of albumin out of the intravascular space in a bovine endothelial cell culture, related to increased exogenously applied shear-stress forces, depends on the thickness of the glycocalyx and its superficial negative charge.
According to these considerations, the glycocalyx plays a fundamental role in the passage of fluid and macromolecules from the intravascular space to the interstitial and intracellular spaces (Fig. 3) (31). With its discovery, the capillary theory proposed by the British physiologist Ernest Starling in 1886 has been reformulated and, to some extent, complemented. In this regard, with the current evidence, it is thought that blood flow in high intravascular pressure blood vessels is maintained, thanks to tight endothelial junctions and the oncotic pressure gradient produced by the glycocalyx by virtue of its negative surface charge. On the other hand, in low pressure blood vessels, there is a highly effective exchange of nutrients and waste products due to the free passage of the plasma constituents toward the cells, thanks to the low hydrostatic and oncotic pressure gradients determined by the glycocalyx (32).
In this regard, some authors, based on modern technology, have suggested that Starling’s Law does not fully explain some physiologic findings such as the fact that there is no venous fluid reabsorption, the interstitial protein concentration has little effect on fluid flux, and the transcapillary flow rate is lower than previously thought (31-33). These findings and the understanding of the role of the glycocalyx in vascular permeability suggest that this theory should be updated, specifically in the following aspects:
Experiments have found that although there is an initial transient capillary response, in which the liquid is seen to be absorbed following a sudden drop in luminal hydrostatic pressure, this changes rapidly again to outward filtration, even in the venous end of the capillary (33).
- The subglycocalyx space is a small protein-free zone located between the glycocalyx and the endothelial cell (Fig. 1). This zone creates a gradient with the luminal osmotic pressure, contrary to what was formerly believed, that there was a gradient between the luminal osmotic pressure and the subglycocalyx osmotic pressure (31, 33). The original Starling theory assumes that the interstitial osmotic pressure is lower than the luminal osmotic pressure. This concept warrants being reevaluated. The physiologic extravasation of plasma proteins through large pores located in the venule segments of the capillaries causes the interstitial osmotic pressure to be very similar to the luminal osmotic pressure. It is suggested that the movement of fluid across the endothelium explained by the classical Starling Principle may be updated to the following formula (9, 33):
where Jv/A is the outward filtration force for a given area, Lp is the membrane hydraulic conductivity (i.e., how hard or easy it is for the liquid to pass through the medium by unit of area transverse to the direction of flow), Pc is the luminal hydrostatic pressure, Pi is the interstitial hydrostatic pressure, σ is the macromolecule reflection coefficient of the membrane, πc is the luminal osmotic pressure, and πg is the subglycocalyx osmotic pressure. This πg is very low with respect to πc; thus, the osmotic pressure gradient is close to πc (9, 33). The endothelial glycocalyx is a significant determinant of hydraulic conductivity because it reduces Lp by mechanically resisting fluid flow. It also affects Lp by being a mechano-transducer of the forces induced by shear-stress which stimulates the endothelial cell to release nitric oxide and alters junctional proteins resulting in an increase in Lp (31, 32).
Glycocalyx Alterations in Sepsis
The pathophysiologic processes in the septic patient are multicausal and quite complex (1). At the microcirculation level, there is often endothelial dysfunction with glycocalyx damage in the initial phases of sepsis (3). The loss of this barrier conditions the activation of coagulation, capillary leakage, and leukocyte contact with the endothelium through the up-regulation of ICAM-1 and VCAM-1 which facilitate neutrophil adherence, clustering and migration out of the intravascular space, in an effort to control the ongoing infectious process. In addition, at the endothelial level, nitric oxide synthase is activated, leading to nitric oxide release with its consequent vasodilation, a characteristic frequently seen in patients with sepsis (4, 18, 27).
In addition, the release of proinflammatory cytokines facilitates the activation of enzymes such as heparanase 1 which plays a fundamental role in the conversion and cleavage of heparan sulfate from the glycocalyx proteoglycans. This enzyme is synthesized as an inactive precursor of 65 kDa which undergoes proteolytic cleavage, yielding 8 and 50 kDa protein subunits that heterodimerize to form an active enzyme, heparanase (38, 39). This enzyme is also implicated in neovascularization, inflammation, and autoimmunity, involving migration of vascular endothelial cells and activated immune system cells. When cleaved, these become smaller molecules (50 kDa) with potent damage-associated molecular patterns (DAMPs) activity (39, 40). These are endogenous signs of damage which are produced during inflammatory processes by live injured cells or on cellular death with a necrotic pattern. Likewise, animal models of lipopolysaccharide-induced sepsis have demonstrated by both electron and intravital microscopy that there is glycocalyx damage and increased vascular permeability, which is associated with elevated glycocalyx injury biomarkers such as syndecan-1 (28).
Likewise, this inflammatory response increases the endothelial cell’s porosity and favors the paracellular movement of albumin toward the interstitial space leading to increased oncotic pressure outside of the intravascular space. This facilitates the development of edema and capillary leakage, a phenomenon frequently seen in septic patients (24). This situation may be magnified depending on the type of liquid used in fluid resuscitation. Clinical studies are needed to evaluate the effect of different crystalloid bolus regimens on glycocalyx integrity. Fluid resuscitation with crystalloids to repair macrohemodynamic disruptions increases the pressure in the intravascular space and contributes to transcapillary fluid leakage which leads to greater interstitial edema, as demonstrated by Bark et al (34) in an animal model. These authors, in a sepsis model, showed that 0.9% saline solution boluses administered in 15 minutes increased plasma volume to a lesser degree than a continuous infusion of 4% albumin over 3 hours, due to its rapid passage toward the interstitial space because of the endothelial dysfunction associated with sepsis (34). These findings suggest that the volume of colloids needed to sustain normovolemia could be lower when administered as a continuous infusion rather than as boluses. The explanation for this could be related to the stabilization and progressive recovery of the glycocalyx triggered by the colloids, which would improve the negative charge of the heparan sulfate optimizing the intravascular oncotic pressure and thus improving microcirculation volume, tissue perfusion pressure, and oxygen delivery to the tissues, as was recently suggested by Torres et al (35) in an animal model of hemorrhagic shock (36).
Similar results have been found in humans related to glycocalyx damage. Steppan et al (26) evaluated glycocalyx abnormalities in postoperative abdominal surgery patients, healthy subjects, and people with a sepsis diagnosis. In a total of 150 individuals, of whom 104 had severe sepsis principally of pulmonary etiology, they found significantly elevated inflammatory mediators such as interleukin-6, ICAM-1, and VCAM-1 compared with the other groups, while postoperative patients only had elevated interleukin-6. However, when they assessed markers suggestive of glycocalyx injury, such as syndecan-1 and heparan sulfate, they found that these were significantly elevated in septic and surgical patients compared with the healthy controls.
The glycocalyx cannot be seen in vivo, which is why special techniques and equipment have been developed to visualize the space between the erythrocytes and the endothelium in sublingual and retinal vessels, thus determining the breadth of the glycocalyx (4, 13). More recently, the measurement of fundamental components of the glycocalyx by enzyme-linked immunosorbent assay techniques (such as syndecan-1, endocan, and heparan sulfate) has been employed (27, 38-40) along with sublingual videomicroscopy measurement. Recently, a model of patients with sepsis showed that there can be glycocalyx involvement without evident damage of other variables that would indicate microcirculation involvement suggesting that the measurement of glycocalyx components may be useful as early biomarkers of endothelial damage in sepsis (27).
THERAPEUTIC STRATEGIES FOR PREVENTING GLYCOCALYX DAMAGE
As has been discussed, the endothelial glycocalyx is injured in patients with inflammatory disorders, especially in patients with sepsis. It is very important to recognize this situation because frequently therapeutic efforts are aimed exclusively at managing the macrocirculation, without taking into account that the microcirculation is very altered (39). In this regard, the consideration of a good, rational resuscitation of this microcirculation based on the ongoing pathophysiologic alterations could contribute to better outcomes.
Research studies have been carried out from different perspectives. Recently, sulodexide has been described, which is a mix of glycosaminoglycan precursors, with 80% heparan sulfate and 20% dermatan sulfate, obtained from porcine intestinal mucosa (28, 41, 42). Sulodexide acts as a heparanase inhibitor, blocks the release of leukocyte matrix metalloproteinases (MMPs), inhibits interleukin-6, stimulates lipoprotein lipase activity and modulates coagulation/fibrinolysis. It is therefore considered to be an anti-inflammatory and antithrombotic medication (41). Gambaro et al (42) have demonstrated that this medication, administered for at least two months, reverses the kidney injuries established in diabetic rat models, increasing the glycocalyx thickness, stability, and function. Recently, Song et al (16), in an animal sepsis model, demonstrated that this medication accelerates the regeneration of the glycocalyx with better control of vascular permeability and improves survival when it is administered 2 hours after inducing the disease.
Research studies on patients with sepsis have focused on taking preventive measures to avoid greater injury because when physicians are faced with this disease, usually the damage to the glycocalyx is already ongoing. In this context, it has been shown that maintaining a good level of plasma proteins, especially albumin, promotes the restoration of the endothelial glycocalyx and the progressive recovery of its functions, thus avoiding greater blood vessel damage due to inflammatory cell adherence and migration (34). An animal model has shown that a low-protein environment produces a complete absence of the endothelial glycocalyx, caused by damage to the MMPs which are responsible for damage to the fundamental components of the glycocalyx. The protective effect of the proteins could be mediated by a substance joined to them, which is a lipid mediator known as sphingosine 1-phosphate. This mediator inhibits MMPs through its receptor located in the cell membrane, preventing endothelial glycocalyx shedding. RBCs and platelets are the main sources of sphingosine-1-phosphate in humans, and it has been demonstrated that, in the absence of albumin, this lipid mediator is released up to 25 times less from these cells (18).
In this regard, studies in animals with 20% albumin infusions have shown that they attenuate glycocalyx damage in models of ischemia/reperfusion of transplanted organs, decreasing interstitial edema, and increased leukocyte adhesion following cold ischemia (29, 43). Likewise, Job et al (44) recently demonstrated, in a bovine endothelial cell model, that the use of exogenous albumin can improve glycocalyx thickness and decrease its stiffness, a phenomenon which in clinical practice can help control the capillary leak syndrome and edema seen in septic patients. Studies in humans are needed to demonstrate that continuous albumin infusions can aid endothelial glycocalyx recovery.
Finally, fluid resuscitation plays a fundamental role in the glycocalyx’s preservation and injury (29, 44). The administration of balanced and unbalanced solution boluses is the cornerstone of the initial treatment of patients with sepsis and is part of the sepsis bundle which has proven to have an important effect on clinical outcomes in various studies (1, 45). The number, composition, and duration of administration of these boluses we consider may be factors which influence the stability of the glycocalyx and may contribute to its injury and be a significant determinant of greater inflammation. This could be one of the possible biological explanations for why crystalloids spend less time in the intravascular space, temporarily recovering tissue perfusion pressure but with limited and transient clinical effects as recently demonstrated Long et al (46). These authors found that following fluid bolus therapy in children with sepsis the cardiac index improved 18% at 5 minutes, but after 60 minutes, it decreased 6% with respect to the baseline, suggesting that its effect was transient and it rapidly passed to the interstitial space. Studies are needed to evaluate the effect of fluid resuscitation in children with sepsis on the endothelial glycocalyx and the clinical consequences which could be associated.
In a normovolemic hemodilution model, Chapell et al (21) recently found that following the administration of a 20 cc/kg bolus of 6% hydroxyethyl starch 130/0.4, there was a 100% increase in atrial natriuretic peptide (ANP) levels. This was significantly associated with an 80% increase in syndecan-1 and hyaluronan, with greater interstitial edema and capillary leakage, suggesting that hypervolemia increases the release of ANP and causes enhanced shedding of the endothelial glycocalyx.
FUTURE DIRECTIONS
The endothelial glycocalyx is a fundamental structural part of the endothelium and is responsible for maintaining the flow and dynamics in perfect equilibrium within the intravascular space. It is affected by acute and chronic inflammation, hyperglycemia and, especially in septic patients, is responsible for capillary leak syndrome. A rapid diagnosis using biomarkers such as syndecan would permit the development of therapeutic strategies aimed at controlling the so-called endothelial failure syndrome, where glycocalyx injury and involvement plays a fundamental role in its clinical spectrum and severity. Studies are needed in patients with severe sepsis and septic shock to determine which medications have a direct effect on glycocalyx recovery and regeneration as well as the effect of balanced and unbalanced solution boluses and endothelial integrity and its relation to clinical outcomes.
ACKNOWLEDGMENT
We thank Dr. Felipe Tirado, MD, PhD, for his valuable contributions in the correction and drafting of this article.
Footnotes
Drs. Fernández-Sarmiento, Salazar-Peláez, and Carcillo conceived the idea for the article. Dr. Fernández-Sarmiento carried out the systematic literature review and designed the figures that represent the glycocalyx. All the authors drafted the article and contributed significantly to the article revision. All authors approved the final article as submitted and agree to be accountable for all aspects of the work. None of the researchers have conflicts of interest to declare.
The authors have disclosed that they do not have any potential conflicts of interest
REFERENCES
- 1.Davis AL, Carcillo JA, Aneja RK, et al. : American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017; 45:1061–1093 [DOI] [PubMed] [Google Scholar]
- 2.Tan B, Ming JJ Sultana R et al. : Global case-fatality rates in pediatric severe sepsis and Septic Shock. A systematic review and meta-analysis. JAMA pediatrics 2019; 4:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Marshall JC, Namendys-Silva SA, et al. ; ICON investigators: Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med 2014; 2:380–386 [DOI] [PubMed] [Google Scholar]
- 4.Nieuwdorp M, Meuwese MC, Vink H, et al. : The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr Opin Lipidol 2005; 16:507–511 [DOI] [PubMed] [Google Scholar]
- 5.Rehm M, Zahler S, Lötsch M, et al. : Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 2004; 100:1211–1223 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg BM, Vink H, Spaan JA: The endothelial glycocalyx protects against myocardial edema. Circ Res 2003; 92:592–594 [DOI] [PubMed] [Google Scholar]
- 7.Van den Berg BM, Nieuwdorp M, Stroes ESG, et al. : Endothelial luminal glycocalyx: Protective barrier between endothelial cells and flowing blood. In: Endothelial Biomedicine. Aird WC (Ed). Leids Universitair Medisch Centrum (LUMC), The Netherlands, Cambridge University Press, 2007, pp 689–695 [Google Scholar]
- 8.Henrich M, Gruss M, Weigand MA: Sepsis-induced degradation of endothelial glycocalix. ScientificWorldJournal 2010; 10:917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levick JR: Capillary filtration-absorption balance reconsidered in light of dynamic extravascular factors. Exp Physiol 1991; 76:825–857 [DOI] [PubMed] [Google Scholar]
- 10.Luft JH: Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 1966; 6:1773–1783 [PubMed] [Google Scholar]
- 11.van den Berg BM, Nieuwdorp M, Stroes ES, et al. : Glycocalyx and endothelial (dys) function: From mice to men. Pharmacol Rep 2006; 58(Suppl):75–80 [PubMed] [Google Scholar]
- 12.Potter DR, Jiang J, Damiano ER: The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res 2009; 104:1318–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donati A, Damiani E, Domizi R, et al. : Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res 2013; 90:86–89 [DOI] [PubMed] [Google Scholar]
- 14.Kolářová H, Ambrůzová B, Svihálková Šindlerová L, et al. : Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014; 2014:694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouverneur M, Berg B, Nieuwdorp M, et al. : Vasculoprotective properties of the endothelial glycocalyx: Effects of fluid shear stress. J Intern Med 2006; 4:393–400 [DOI] [PubMed] [Google Scholar]
- 16.Song JW, Zullo JA, Liveris D, et al. : Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther 2017; 1:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappell D, Westphal M, Jacob M: The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol 2009; 2:155–162 [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Adamson RH, Curry FR, et al. : Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol 2014; 306:H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mochizuki S, Vink H, Hiramatsu O, et al. : Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 2003; 2:722–726 [DOI] [PubMed] [Google Scholar]
- 20.Wang W: Change in properties of the glycocalyx affects the shear rate and stress distribution on endothelial cells. J Biomech Eng 2007; 129:324–329 [DOI] [PubMed] [Google Scholar]
- 21.Chappell D, Bruegger D, Potzel J, et al. : Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 2014; 18:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Haaren PMA, VanBavel E, Vink H, et al. : Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physio. 2003; 6:2848–2856 [DOI] [PubMed] [Google Scholar]
- 23.Tarbell JM, Pahakis MY: Mechanotransduction and the glycocalyx. J Intern Med 2006; 4:339–350 [DOI] [PubMed] [Google Scholar]
- 24.Ueda A, Shimomura M, Ikeda M, et al. : Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am J Physiol-Heart Circ Physiol 2004; 5:2287–2294 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt EP, Yang Y, Janssen WJ, et al. : The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18:1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfieri A, Watson J, Kammerer R, et al. : Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Crit Care 2012, 16:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steppan J, Hofer S, Funke B, et al. : Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res 2011; 1:136–41 [DOI] [PubMed] [Google Scholar]
- 28.Rovas A, Seidel LM, Vink H, et al. : Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit Care 2019; 23:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kataoka H, Ushiyama A, Akimoto Y, et al. : Structural behavior of the endothelial glycocalyx is associated with pathophysiologic status in septic mice: An integrated approach to analyzing the behavior and function of the glycocalyx using both electron and fluorescence intravital microscopy. Anesth Analg 2017; 125:874–883 [DOI] [PubMed] [Google Scholar]
- 30.Hippensteel JA, Uchimido R, Tyler PD, et al. : Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care 2019; 23:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob M, Bruegger D, Rehm M, et al. : The endothelial glycocalyx affords compatibility of Starling’s principle and high cardiac interstitial albumin levels. Cardiovasc Res 2007; 73:575–586 [DOI] [PubMed] [Google Scholar]
- 32.Woodcock TE, Woodcock TM: Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth 2012; 3:384–394 [DOI] [PubMed] [Google Scholar]
- 33.Levick JR, Michel CC: Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 2010; 87:198–210 [DOI] [PubMed] [Google Scholar]
- 34.Bark BP, Persson J, Grände PO: Importance of the infusion rate for the plasma expanding effect of 5% albumin, 6% HES 130/0.4, 4% gelatin, and 0.9% NaCl in the septic rat. Crit Care Med 2013; 41:857–866 [DOI] [PubMed] [Google Scholar]
- 35.Torres LN, Chung KK, Salgado CL, et al. : Low-volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care 2017; 21:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milford E, Reade M: Resuscitation fluid choices to preserve the endothelial glycocalyx. Crit Care 2019; 23:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehm M, Bruegger D, Christ F, et al. : Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116:1896–1906 [DOI] [PubMed] [Google Scholar]
- 38.Oshima K, Haeger S, Hippnsteel J: More than a biomarker: the systemic consequences of heparan sulfate fragments released during endothelial surface layer degradation. Pulm Circ 2017; 1:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilan V, Naggi I, Neta A, et al. : Heparanase: Structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des 2007; 13:2057–2073 [DOI] [PubMed] [Google Scholar]
- 40.Martin L, De Santis R, Koczera P, et al. : The synthetic antimicrobial peptide 19-2.5 Interacts with Heparanase and heparan sulfate in Murine and human sepsis. PLoS One 2015; 10:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li R, Xing J, Mu X, et al. : Sulodexide therapy for the treatment of diabetic nephropathy, a meta-analysis and literature review. Drug Des Devel Ther 2015; 9:6275–6283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gambaro G, Venturini AP, Noonan DM, et al. : Treatment with a glycosaminoglycan formulation ameliorates experimental diabetic nephropaty. Kidney Int 1994; 3:797–806 [DOI] [PubMed] [Google Scholar]
- 43.Jacob M, Paul O, Mehringer L, et al. : Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation 2009; 7:956–965 [DOI] [PubMed] [Google Scholar]
- 44.Job KM, O’Callaghan R, Hlady V, et al. : The biomechanical effects of resuscitation colloids on the compromised lung endothelial glycocalyx. Anesth Analg 2016; 123:382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Sarmiento J, Carcillo JA, Salinas CM, et al. : Effect of a sepsis educational intervention on hospital stay. Pediatr Crit Care Med 2018; 19:e321–e328 [DOI] [PubMed] [Google Scholar]
- 46.Long E, Babl FE, Oakley E, et al. Cardiac Index changes with fluid bolus therapy in children with sepsis- An observational study. Pediatr Crit Care 2018; 6:513–551 [DOI] [PubMed] [Google Scholar]