Abstract
Just over 1 year following rollout of the first vaccines for coronavirus disease 2019, 572 million doses have been administered in the United States. Compared with the number of vaccines administered, adverse effects such as anaphylaxis have been rare, and seemingly, the more serious the effect, the rarer the occurrence. Despite these adverse effects, there are few, if any, true contraindications to coronavirus disease 2019 vaccination and most individuals recover without further sequelae. This review provides guidance for the allergist/immunologist regarding appropriate next steps based on patient’s known allergy history or adverse reaction after receipt of coronavirus disease 2019 vaccine to assist in safe global immunization.
Key words: COVID-19, vaccines, anaphylaxis, allergic reaction, adverse effects
Case Description
A 31-year-old White woman with a history of chronic idiopathic urticaria on antihistamines was evaluated in our outpatient drug allergy clinic after experiencing an immediate reaction to her first dose of the Pfizer-BioNTech messenger RNA (mRNA)-based coronavirus disease 2019 (COVID-19) vaccine. Forty-five minutes after the vaccination, she developed generalized urticaria, nausea, and lightheadedness. She was seen at the local emergency department, treated with diphenhydramine with symptomatic improvement, and discharged home. Serum tryptase level drawn 7 hours after the reaction onset was 2.2 μg/L. After her initial reaction, she was evaluated in our outpatient drug allergy clinic. At this evaluation, the patient was not aware of previous intolerances to polyethylene glycol (PEG)-containing products. She had negative prick and intradermal skin testing results to standard doses of PEG and polysorbates. This was followed by an oral challenge with PEG3350 in the form of Miralax, in which the patient had no symptoms during 1.5 hours of observation. A week later, she received the second dose of the Pfizer-BioNTech COVID-19 vaccine with the recommended 0.3-mL injection as a single dose intramuscularly following premedication with cetirizine 10 mg twice a day and tolerated the vaccine well.
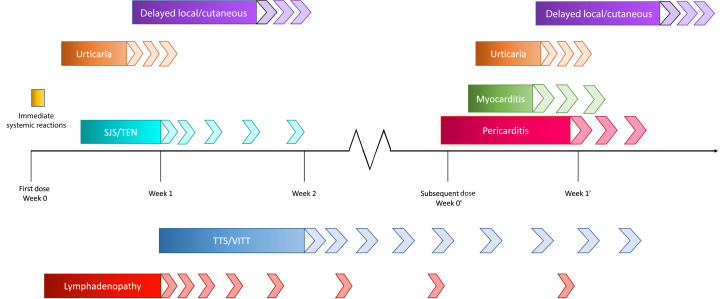
We describe this case to highlight the tolerance of a second dose of an mRNA vaccine despite a first-dose reaction. The following review further summarizes the important immune-mediated and additional adverse reactions associated with COVID-19 vaccines (Table I and Figure 1 ), potential mechanisms, and the steps forward in light of these adverse effects.
Table I.
Summary of rates, selected clinical features, and proposed actions for adverse events highlighted in Figure 1
| Adverse event | Vaccine(s) implicated | Estimated rate of effect | Average time to onset | Average duration of symptoms | Actions/considerations |
|---|---|---|---|---|---|
| Immediate systemic reactions | All, but most prominent with mRNA vaccines | Estimates vary but anywhere between 2 and 5 per 1 million and 7.9 per 1 million | 5-60 min after vaccination | 24 h, typically less |
|
| Acute urticaria | mRNA vaccines | 20,000 per 1 million | >24 h after vaccination | Variable, often within 2 wk of onset but possibly longer |
|
| Delayed cutaneous reactions | mRNA vaccines | 7 d after vaccination | 3-5 d, shorter if occurring with second dose |
|
|
| Myocarditis | mRNA vaccines | 40.6 per million (males 12-29 y); 4.2 per million (females 12-29 y); 2.4 per million (males >30 y); 1 per million (females >30 y) | 3-4 d after vaccination, typically with the second (or subsequent) dose but can occur after first dose | Acute symptoms for 3-5 d, then complete resolution over days to weeks |
|
| Thrombosis with thrombocytopenia syndrome/VITT | Adenovirus vector vaccines | 3.8 per million; mortality of up to 40% of individuals with diagnosis | Usually within 1-2 wk but up to 42 d after vaccination | Variable, sequelae can be long lasting |
|
AGEP, Acute generalized exanthematous pustulosis; NSAID, nonsteroidal anti-inflammatory drug; SJS/TEN, Stevens-Johnson syndrome or toxic epidermal necrolysis.
Figure 1.
Timeline of adverse reactions. The chart displays the time frame in which an individual is at highest risk of experiencing symptoms, with each of the adverse reactions discussed. It is important to note that TTS/VITT typically occurs within 1 to 2 weeks of vaccination, but the diagnostic criteria state that this may be considered for diagnosis up to 42 days after receipt of immunization. In addition, myocarditis and pericarditis may occur after first dose. SJS/TEN, Stevens-Johnson syndrome or toxic epidermal necrolysis; TTS, thrombosis with thrombocytopenia syndrome.
Background
In the midst of the COVID-19 pandemic, there has been a rapid global vaccination deployment and response, with more than 11.5 billion doses administered worldwide as of April 25, 2022.1 The mRNA vaccines were developed with unprecedented speed and safety, with both the Pfizer-BioNTech and Moderna vaccines receiving emergency use authorization by December 2020. In the United States, this was soon followed by a single-dose adenoviral vector vaccine developed by Johnson and Johnson (J&J, Janssen). Contrary to general beliefs, the technology surrounding mRNA vaccines and therapeutics was not new, and several decades had already been spent developing such technology, with vaccines in early-phase clinical trials for diseases of global importance such as HIV and Zika viruses,2 , 3 promising new vaccines for cancer,4 as well as therapeutics independent of vaccines.5 Despite this, the population was unfamiliar with mRNA vaccine technology, because this was the first time mRNA vaccines had been used in a mass vaccination strategy. Impressively, 2 mRNA vaccines were approved and deployed for emergency use within less than 12 months of the arrival of COVID-19 to the United States. In Europe and the United States, within the initial 2 weeks of rollout of the mRNA vaccines, several cases of suspected anaphylaxis were reported; however, current information supports that the vaccines have been overwhelmingly safe, with comparatively few adverse events to the large scale of vaccines administered.6 In addition to the 2 mRNA vaccines (Pfizer-BioNTech and Moderna) and the 3 adenoviral vector vaccines (J&J, Astra Zeneca, and Sputnik) (Table II ), inactivated vaccines have been deployed in China and other parts of Asia.7, 8, 9, 10, 11, 12, 13 Thromboembolic events and thrombosis with thrombocytopenia, though rare, have been associated with the administration of the Astra Zeneca (ChAdOx1 nCoV-19/AZD1222) and J&J (Ad26.COV2.S) adenoviral vector vaccines and are described further in the latter portion of the text. To date this has not been a signal with other viral vector vaccines such as the Sputnik V (Gam-COVID-Vac).14 At the time of this review, more than 572 million doses of COVID-19 vaccines have been administered in the United States.1 , 15
Table II.
Summary of currently approved vaccines in the United States and the United Kingdom
| Characteristics/administration | mRNA vaccines | Inactive viral vector vaccines |
|---|---|---|
| History |
|
|
| Pfizer-BioNTech | Moderna | Johnson & Johnson (J&J, Janssen) | Oxford/AstraZeneca | |
|---|---|---|---|---|
| Also known As | BNT162b2 Comirnaty |
mRNA-1273 Spikevax |
Ad26.COV2.S | ChAdOx1 nCoV-19/AZD1222 Covishield; Vaxzervia |
| Composition | PEG2000 nanoparticle formulated nucleoside-modified mRNA encoding the spike protein of SARS-CoV-2 | Single strand of mRNA encoding the spike protein of SARS-CoV-2 encapsulated in a PEG2000 lipid nanoparticle carrier | Recombinant, replication-deficient human adenovirus type 26 vector encoding SARS-CoV-2 spike protein | Replication-deficient chimpanzee adenovirus vector that is modified to encode the spike protein of SARS-CoV-2 |
| Recommended administration | Two 0.3-mL (adult) or 0.2-mL (child) IM doses separated by at least 21 d | Two 0.5-mL IM doses separated by at least 28 d | Single 0.5-mL IM dose | Two 0.5-mL IM doses separated by 8-12 wk |
| Booster administration | 0.3-mL IM dose at least 5 mo after primary series for those ≥12 y; no booster for children | 0.25-mL IM dose at least 5 mo after primary series | Vaccination with either Pfizer (0.3 mL) or Moderna (0.25 mL) vaccines encouraged at least 2 mo after initial J&J vaccine | In the UK, vaccination with either Pfizer (0.3 mL) or Moderna (0.25 mL) vaccine at least 3 mo after primary series |
IM, Intramuscular.
Spectrum of Reactions and Differential Diagnosis
Immediate systemic reactions
Immediate systemic reactions are those that can be described by acute onset (within 4 hours of vaccination) of generalized symptoms such as rash, flushing, swelling, or difficulty breathing, among others; these may also be referred to as immediate hypersensitivity reactions or immediate allergic-like reactions. Since the emergency approval of the mRNA vaccines, immediate hypersensitivity reactions were initially described at rates of 2.5 to 11.1 per 1 million vaccine doses administered.16 Obtaining a true number of immediate systemic reactions has been difficult due to the variation in reports, lack of physician confirmation and a standard definition of “anaphylaxis,” and ability for self-reporting, but the real-world data seem to suggest rates that are slightly higher than the historical rates across all vaccines (about 1.3 per 1 million doses).17 , 18 The Centers for Disease Control and Prevention (CDC) suggests rates between 2 and 5 per 1 million,19 a large population-based study suggests a rate of 4.8 per 1 million,20 and a recent meta-analysis describes a rate of 7.91 per 1 million vaccine doses administered.21 Although the mechanism behind the high rate of immediate reactions currently remains elusive, evidence to date supports a non-IgE mechanism, because we can be reassured that, across various studies, most individuals with first-dose immediate reactions to an mRNA vaccine have either tolerated subsequent doses without symptoms or had very mild symptoms not indicative of an IgE mechanism.22, 23, 24, 25, 26, 27, 28 Further support comes from a meta-analysis encompassing more than 1300 individuals with first-dose reactions that demonstrated that, of these individuals, only 13% went on to develop reactions at second dose, with less than 0.5% being categorized as severe reactions.29 Many conceivable mechanisms have been proposed including direct mast cell activation, whether IgE- or non–IgE-mediated, complement activation, and contact system activation by nucleic acids.30 It is important to note that much of the data currently available lack the laboratory studies needed to discern mechanism of the reaction, and randomized double-blind studies are ongoing in an attempt to elucidate the mechanism of immediate reactions associated with mRNA vaccines.17 , 22 , 30, 31, 32 The construct or antigen that might be driving these immediate reactions, regardless of the mechanism, could be an inactive component of the vaccine such as PEG2000 used to stabilize the lipid nanoparticle, an active component of the vaccine such as the mRNA, or immune response to the translated spike protein itself.
Individuals experiencing immediate reactions commonly reported symptoms of pruritus, flushing, rash, difficulty breathing, and the sensation of throat tightness with onset minutes after immunization; some experienced disorientation and dizziness.33 Although IgE-mediated allergy and clinical anaphylaxis are the most serious considerations given these symptoms, as mentioned, tolerance of the second dose in most individuals is not consistent with this. Therefore, although the mechanism is unclear, the tolerance of the second dose makes an IgE mechanism much less likely in most patients, and other etiologies including complement activation, paradoxical vocal cord motion or laryngospasm, anxiety or panic attacks, and vasovagal responses need to be considered.33 , 34
Although likely very rare, true IgE-mediated allergic reaction to mRNA-based COVID vaccines is possible, though seemingly unlikely on the basis of the information we have gained in the last year. Previously, it was proposed that the mechanism surrounded sensitization toward PEG present in the carrier of the mRNA in these vaccines.30 , 35 , 36 Initially a significant unknown, the implications for individuals with true IgE-mediated reactions to PEG—such as PEG3350 in steroids or bowel preparations—is becoming clearer with the accumulation of information. At minimum—even with polysorbate 80 cross-reactivity—adenoviral vector vaccines may be safe for PEG-allergic individuals,35 , 37 , 38 though this alternative has not been directly compared with risk with mRNA vaccination and leaves unanswered questions of efficacy.21 Data suggest that patients with immediate reactions to pegylated compounds such as PEG-asparaginase are tolerant of mRNA vaccines.39 , 40 Patients in these reports had largely tolerated PEG3350 laxatives following their immediate reactions to PEG-asparaginase. Evolving evidence suggests that mRNA vaccination may even be safe for those with a confirmed or highly suspected IgE-mediated PEG allergy, as demonstrated in a small case series of individuals with PEG allergy who tolerated mRNA vaccination and in a small study in which individuals with PEG allergy unknowingly received mRNA vaccination.38 , 41 In the case of hesitancy or anxiety of the patient or provider, an alternative to full vaccination may include graded vaccine dosing, which has been successfully implemented with administration of the vaccine in increasing doses given every 10 to 15 minutes depending on the protocol.42 , 43 However, it should be noted that the efficacy of these protocols has not been compared with full vaccination, and it is not known whether graded administration decreases the risk for immediate reactions.
Complement activation can mimic immediate hypersensitivity in the form of complement activation–related pseudoallergy, and PEG and liposomes can be implicated in this pathway.30 , 44 Historically, these reactions have occurred with intravenous preparations, and complement activation–related pseudoallergy reactions due to intramuscular medications or vaccines would be a novel, though possible, finding. Patients may develop symptoms of anaphylaxis including angioedema, wheezing, urticaria, and syncope, among many other symptoms, which can be confused with an immediate IgE-mediated reaction.45 In the acute setting, it is difficult to differentiate between potential diagnoses. Obtaining a serum tryptase level between 30 and 90 minutes of symptom onset along with screening terminal (C5-C9) complement levels can be helpful in determining an IgE- or complement-mediated etiology, respectively.34 Both of these laboratory evaluations are available commercially; however, total complement may be an acceptable—but less sensitive—alternative when terminal complement cannot be obtained. With isolated cutaneous reactions, adjuncts including oral, nonsedating antihistamines may prove helpful in providing symptomatic relief while the patient remains under close observation. However, systemic steroids are generally not recommended because of their relative lack of efficacy in treatment and potential to blunt the immune response to vaccination.21
With initial reports of immediate systemic reactions to mRNA vaccines and the concern for PEG as a possible inciting agent, it was recommended that high-risk individuals have prevaccination screening with or without skin testing to PEG and polysorbate excipients.34 As evidence has evolved, it has become clear that the role of skin testing is more limited than originally thought, with emphasis on history as an important guide for vaccination, especially in individuals with first-dose reaction history.21 , 28 Studies have highlighted the inability of skin testing to reliably predict clinical allergy to PEG and related excipients due to high rates of false positives and the increasing idea that a non–IgE-mediated mechanism is responsible for clinical reaction.25 , 28 There exist infrequent cases when skin testing may be helpful in guiding further vaccine administration. For example, individuals with significant vaccine hesitancy who would otherwise forfeit initial or subsequent vaccination may benefit from limited skin testing to PEG products with protocols outlined in the literature.34 , 46 However, an individualized approach and shared decision making, along with counseling and discussion, should be implemented given the knowledge that most individuals with a history of immediate reactions after the first dose of mRNA vaccine have tolerated subsequent vaccination,23 , 24 , 26 , 28 , 29 that PEG-allergic individuals have tolerated mRNA vaccination,38 , 41 and the potential for false positives with skin testing that may further complicate vaccination.21 In the overwhelming majority of cases, skin testing is unlikely to provide benefit in facilitating vaccination.
When to investigate immediate systemic reactions to the COVID-19 vaccines?
In the first few months following the reports of anaphylaxis, there was an abundance of caution in terms of management of patients because it was not known whether these were IgE-mediated or whether reactions could worsen on subsequent doses. Currently, we know that most patients will tolerate subsequent doses, which strongly suggests that most immediate vaccine reactions are not IgE-mediated. In addition, many of the patients who reacted on first dose have gone on to receive booster vaccination at least 6 months later, which suggests that original thoughts of an IgE mechanism with refractory period of mast cell degranulation between first and second doses is unlikely. Several articles have now been published supporting the safety of subsequent doses even in the setting of anaphylaxis with the first dose.23 , 24 , 26 , 29 In addition, current guidelines support the idea that excipient testing does not contribute to vaccine safety and could, in theory, delay vaccination in individuals with a history of allergic-like reactions.21 False positives have been described with specific PEG and polysorbate reagents particularly in the setting of intradermal testing.28 Moreover, the most recent evidence suggests that known small samples of PEG-allergic individuals can tolerate mRNA-based vaccines.38 , 41 For this reason, it should be a shared patient decision to investigate a systemic allergic reaction, and vaccination should not be withheld or delayed in favor of excipient skin testing and allergist evaluation except in rare circumstances.21
Delayed hypersensitivity reactions
As with most vaccines, the COVID-19 mRNA vaccines have been associated with many different clinical phenotypes of delayed drug rashes. A retrospective study of 414 patients with cutaneous reactions after mRNA COVID-19 vaccination revealed that most patients experienced cutaneous symptoms after the second dose (63%) compared with the first dose (21%) and with both doses (16%).47 After the Moderna vaccine, delayed large local reactions were most common, and with Pfizer, urticaria was the most common cutaneous finding.47 A more recent schema has supported that delayed reactions to COVID-19 vaccines be classified as papulosquamous, papulovesicular, and pityriasis rosea-like.48 Consistent with other reports, these typically occur more than 4 hours after vaccination and none of the 414 patients experienced severe sequelae; less than half of the individuals who experienced symptoms after the first dose had similar symptoms following the subsequent dose in series.47 In a similar study of more than 40,000 employees of a large hospital system, 4% of individuals reported cutaneous symptoms, with rash and pruritus being the most common.49 In all cases, symptoms were self-limited and more than 80% of individuals who had cutaneous symptoms after the first tolerated the second dose without a similar reaction.49 These studies support the idea that cutaneous reactions are self-limited and, in general, are not a contraindication to repeat vaccination.
Delayed rashes
Diffuse, delayed morbilliform eruptions have been described in few individuals receiving the mRNA-based COVID-19 vaccines.47 , 50 , 51 These reported cases typically have occurred within 48 hours following first-dose vaccination, resolve over 1 week without sequelae, and most tolerate dose 2.47 , 51 According to 2 studies, morbilliform eruptions were more common in individuals receiving the Pfizer vaccine.47 , 51 These rashes are generally not cause for concern and likely do not represent an allergic reaction, but rather, an effect of host immune response to the vaccine.47
Rare reports of severe cutaneous adverse reactions have been associated with both COVID-19 infection and COVID-19 vaccination. Given the background rate of these reactions in the community and the background rate of COVID-19 disease, it would be difficult to prove causality. A past history of a severe cutaneous adverse reaction—such as Stevens-Johnson syndrome or toxic epidermal necrolysis, acute generalized exanthematous pustulosis, or drug reaction with eosinophilia and systemic symptoms—related to another drug or vaccine is not a contraindication to receiving a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine and is likely much safer than the risk of natural infection sequelae with COVID-19. Although erythema multiforme has been more prevalently described, SJS-like illness has been rarely reported, with symptom onset 3 to 5 days following first- or second-dose vaccination, resolution over 10 to 14 days, and apparent response to cyclosporine or prednisone.52 , 53 In these cases, it has not clearly been delineated whether this could have been due to underlying COVID-19 infection, which has been associated with erythema multiforme major. Acute generalized exanthematous pustulosis has been reported after viral vector54 , 55 and mRNA vaccines.56 The described cutaneous eruptions had the classic pustular appearance involving the intertriginous areas and spreading thereafter, desquamated after several days, and resolved without event.54, 55, 56 Consideration for future COVID-19 vaccination after a severe cutaneous adverse reaction should be on a case-by-case basis, because causality is often difficult to assess.
Delayed local reactions
Many individuals receiving COVID-19 mRNA-based vaccines have reported delayed, large local reactions, which have come to be known as “COVID Arm.” These delayed reactions are characterized by any combination of erythema, induration, or tenderness occurring approximately 7 days after first mRNA vaccine, lasting an average of 4 to 5 days; there is lesser time to occurrence (2 days) and resolution (3 days) when occurring after second-dose vaccination in both the Moderna and Pfizer vaccines.47 , 57 , 58 The incidence of COVID Arm is unclear at this time, but 1 case series, done exclusively in the Pfizer vaccine, revealed delayed local reactions in 2.1% of individuals.59 Recurrence of delayed, large local reactions is variable, with reports of anywhere from 32% to 75% of individuals who reported symptoms with the first dose.57, 58, 59
Delayed injection-site reactions after immunizations are not new. Previously, there have been reports of delayed-type hypersensitivity reactions to vaccines containing adjuvants such as alum.47 , 60 The large, local reactions associated with mRNA vaccines have a histopathology and immunohistochemistry pattern in keeping with delayed-type hypersensitivity on limited samples, though it is unclear whether this is the true pathology.57 Skin biopsies for delayed reactions associated with COVID-19 vaccination are not necessary and are reserved for atypical or refractory cases. Similarly, skin testing is not indicated and does not predict those individuals who may have a delayed local reaction; in fact, evidence has shown that individuals who previously tolerated vaccination or were previously unexposed to vaccination all developed local edema and erythema at the site of intradermal testing, possibly reflecting protective immune response to the vaccine rather than a pathologic immune response.61 Many patients may appear as though they have a superinfection of the injection site and are treated with oral antibiotics for bacterial cellulitis; however, antibiotics have not been shown necessary for resolution.47 , 57 , 58 As has been concluded with additional cutaneous reactions, the development of COVID Arm after vaccination is self-limited and does not exclude the individual from further immunization with mRNA COVID-19 vaccinations.
Delayed urticaria/angioedema
Specific analysis of vaccine-related urticaria revealed that it is most often experienced in a delayed fashion (>24 hours after vaccination) and is more likely to occur with the Moderna vaccine than with the Pfizer vaccine.47 , 49 Studies have revealed an estimated incidence of urticaria of 1% and of angioedema of 0.7%, occurring more often with the first dose of the vaccine.49 In patients who experienced urticaria or angioedema with the first dose, less than 5% went on to develop recurrent symptoms on repeat vaccination.49 It is thought that delayed urticaria and angioedema symptoms after mRNA vaccination represent host immune response to vaccination rather than an allergic type of reaction to the vaccine or an excipient component.47 Delayed urticaria and/or angioedema should implore the provider to investigate any additional history that might suggest preexisting chronic urticaria, and evaluation may include complement studies to investigate delayed complement activation. Delayed urticaria is established to be associated with immune stimulation and mast cell activation independent of IgE, as is the case with infectious processes (often viral) and some immunizations.18 In cases of histamine-mediated urticaria and/or angioedema, individuals respond well to oral histamine receptor (H)1 and H2 blockers for symptom control while the urticaria self-resolves within weeks. In individuals who are predisposed to development of urticaria and/or angioedema, it may be beneficial to recommend premedication with oral antihistamines in the 48 to 72 hours preceding vaccination with mRNA vaccines to mitigate this uncomfortable immune response. As has been supported by clinical studies, isolated urticaria/angioedema should not be a contraindication to further immunization with mRNA vaccines.47 , 49
Miscellaneous cutaneous reactions
SARS-CoV-2 mRNA and viral vector vaccination, as well as natural infection, has been proposed to be associated with additional cutaneous findings. Specifically, varicella zoster virus (VZV) reactivation has occurred a median of 5 days following vaccination in some individuals, though rates are inconsistent, and other, larger analyses have suggested a lack of clear evidence of reactivation after vaccination.62 Of the case reports supporting reactivation, the ages and medical conditions of the individuals varied, though 2 individuals had a remote history of immunosuppression that was not prescribed or relevant at the time of VZV diagnosis.63 , 64 Onset of painful, vesicular skin lesions was within 1 week of immunization and was not accompanied by any additional symptoms63 , 64; 1 patient did have postherpetic neuralgia for about 1 month after initial symptoms that later resolved.65 It is thought that some degree of immunomodulation by the vaccine is contributing to the reactivation of virus64, 65, 66, 67; in addition, there have been reports of VZV reactivation in those with COVID-19 infection that is thought to be due to transient T-cell lymphopenia caused by the virus, though it is not clear that this mechanism holds true for vaccination-related VZV.64 A large study of real-world adverse effects revealed that VZV reactivation was 1 of 3 adverse effects that was higher in those vaccinated versus the unvaccinated (risk difference, 15.8 events per 100,000).6 The additional adverse events greater in the vaccinated are myocarditis (risk difference, 2.7 events per 100,000) and lymphadenopathy (risk difference, 78.4 events per 100,000), both of which are discussed in further detail later.6 On the contrary, a retrospective cohort study with historical and contemporary controls found no difference in the risk ratio of herpes zoster between vaccinated and unvaccinated individuals (0.91 for historical and 0.98 for contemporary).62 Interestingly, patients who have received dermal fillers are at risk for worsening of swelling at the site of the filler injection with both mRNA COVID-19 vaccination and natural infection with COVID-19. The mechanism of edema is thought to be activation of a local immune response and is not a reason to withhold or delay subsequent vaccination.68
Myocarditis/pericarditis
Reports of myocarditis and pericarditis have been some of the more concerning events that have occurred at rates higher than what would be expected in the general population, particularly in young males in whom the baseline rates of myocarditis were already higher.6 The rates of myocarditis vary on the basis of age group and sex; a large study of Vaccine Adverse Event Reporting System reports in the United States highlights the difference in reporting between age groups and sex, with younger individuals having reporting rates between 50 and 100 per 1 million for males and between 6 and 10 per 1 million in females.69 Reports from the CDC have highlighted the association between COVID-19 infection and myocarditis, citing that individuals with true COVID infection have 16 times the risk of associated myocarditis when compared with myocarditis in those without COVID-19 infection.70 A large real-world study highlights that true SARS-CoV-2 infection is associated with a higher rate of myocarditis than in vaccination, with a risk difference of 11 per 100,000 in those with COVID-19 infection,6 and it is important to consider that infection-associated cases tend to be more severe in nature. Another comparison in the same study supports that myocarditis seems to be higher in the vaccinated versus the unvaccinated general population, with a risk difference of 2.7 events per 100,000.6 Synthesis of this information together supports that infection-associated myocarditis is more common than vaccine-associated myocarditis but that vaccine-associated myocarditis does occur. It is also important to note the age and sex preference for vaccine-associated myocarditis cases in the adolescent and young adult male population,69 the mild nature of cases, and the relatively rapid resolution without intervention71, 72, 73 compared with the cases of myocarditis that have occurred with COVID-19 infection.
The reported myocarditis cases indicate that young, generally healthy males seem to be at the greatest risk for myocarditis, with a single case series reporting events in adolescents as young as age 15 years.72 , 74 Presenting symptoms include severe chest pain that can be associated with fever that occurs, on average, 4 days after immunization and more commonly after the second or subsequent doses of mRNA vaccines74; events may be associated more with the Moderna vaccine over the Pfizer vaccine, with one study reporting hazard ratios of 3.92 and 1.34, respectively,75 and another study showing reporting rates for Moderna vaccine 5.1 times higher than for the Pfizer vaccine.76 Patients are noted to have electrocardiogram abnormalities and troponin elevation along with cardiac magnetic resonance imaging findings of inflammation, and there were no additional plausible causative agents identified.71 , 72 , 74 Although the reason remains unclear, there does seem to be an association between COVID-19 mRNA vaccines and increased risk for myocarditis, but clinical studies and further evaluation have supported safe and effective vaccination, especially in adolescents and children in the general population, given the comparatively low risk of side effects.77 Recurrent myocarditis is very rare in the general population. Currently, there are no known cases of recurrent vaccine-associated myocarditis following an initial episode of vaccine-induced myocarditis; however, there are some case reports of vaccine-associated myocarditis following initial virus-induced myocarditis, including SARS-CoV-2, with full recovery in all cases.78 , 79
There have also been reports of pericarditis without evidence of myocardial inflammation after first or subsequent doses of SARS-CoV-2 vaccine. A large study of 40 hospitals in the United States followed more than 2 million patients after at least the first dose of COVID-19 vaccine and reported a larger number of cases of pericarditis37—compared with myocarditis cases20—occurring after either the first dose or the second dose.80 Pericarditis cases differed from myocarditis cases because the median age was 59 years and average time to onset was 20 days after receipt of vaccine.80 None of the 37 patients experienced severe sequelae.80 When compared with data from previous years during the same time period, the data from this study found a significant increase in the incidence of cases of both myocarditis and pericarditis80; however, large-scale population analysis has shown only myocarditis, not pericarditis, with a higher rate in vaccinated individuals.6
Providers should consider the diagnosis of myocarditis in individuals who report severe chest pain accompanied by fever or shortness of breath in the few days after receipt of an mRNA-based COVID vaccine. With some outliers, the demographic characteristics have revealed that a higher index of suspicion should occur in male patients in the late adolescent or young adult age group. In patients outside of the expected age range and in those predisposed to acute coronary events, pericarditis and ST-segment elevation myocardial infarction should be considered as part of the differential diagnosis, respectively.
For the patients diagnosed with postvaccine myocarditis, treatment is largely supportive, and individuals have recovered without sequelae.74 Most individuals received nonsteroidal anti-inflammatory drugs alone while under close observation, whereas others did receive colchicine or steroids in addition to nonsteroidal anti-inflammatory drugs.71, 72, 73, 74 The safety of administering further doses of the mRNA vaccines after acute myocarditis has not been investigated on a large scale; however, 1 case series reported subsequent doses administered in 2 patients having recovered from myocarditis and 7 patients recovered from pericarditis without recurrence of symptoms.80 Additional descriptive studies have shown that there may be a decreased risk of cardiac inflammation with increased time between first and subsequent doses (eg, >56 days),76 supporting a delay in subsequent vaccination with mRNA vaccine in those with positive cases. Currently, there do not exist clear recommendations regarding further COVID vaccination in these individuals, and vaccination is, in general, deferred until we have more information, but shared decision making between the patient and the provider may be used.
The lack of myocardial biopsies in the cases of mRNA vaccine–associated myocarditis reported limits the ability to study the inflammation. Current theories posit an antibody-mediated mechanism, as is thought to be the mechanism behind virus-induced myocarditis in viruses other than COVID.81 Regardless of mechanism, it is important for providers to consider myocarditis and pericarditis in patients with chest pain and electrocardiogram abnormalities after mRNA vaccines.
Thrombosis with thrombocytopenia syndrome
Thrombosis with thrombocytopenia syndrome, also termed vaccine-induced immune-mediated thrombosis and thrombocytopenia (VITT), has been associated with the J&J and AstraZeneca adenovirus vector vaccines—not other adenovirus vaccines such as Sputnik V. In addition to the viral vector vaccines, there have been rare thrombotic complications described with mRNA vaccines,82 though it may be difficult to discern causality in these cases, because these may be spontaneous in nature and coincide with mRNA vaccine receipt given the small number of reports compared with J&J vaccines. This continues to be a rare finding, with current rates estimated to be about 3.8 per 1 million J&J vaccine doses administered in the United States (14,080,087 total J&J doses administered).83 The incidence does increase with age up until women have reached menopause, and women in the fifth decade of life have a rate of 9.0 per 1 million J&J vaccine doses (of 1,108,495 total J&J doses in this age group).83 Although the pathogenesis of the disease is not clear, it is thought to be similar to heparin-induced thrombocytopenia, because patients have similar clinical characteristics and the presence of anti–platelet factor 4 antibodies, which are pathognomonic for heparin-induced thrombocytopenia84, 85, 86; however, the exogenous trigger of heparin has been absent in all these patients, suggesting a yet-to-be-determined antigen complex as a trigger and the categorization of VITT similar to autoimmune heparin-induced thrombocytopenia.84 , 87 Table III summarizes the features, diagnosis, and therapeutics of VITT.85 , 88, 89, 90 Although the incidence seems to be quite rare, the diagnosis is of particular concern due to associated mortality rates, as high as 40%, secondary to complications from thrombosis; in CDC reports, this has equated to a fatality rate of 0.6 per 1 million J&J vaccines administered (of >14 million total J&J doses).83
Table III.
Summary of thrombosis with thrombocytopenia syndrome
| Associated vaccines | Incidence | Presenting features | Evaluation | Diagnostic criteria | Associated risk factors | Potential therapeutics |
|---|---|---|---|---|---|---|
| J&J/Janssen and AstraZeneca | 3.8 per million vaccine doses administered |
|
Based on presenting symptoms— may include extremity ultrasound, CT venogram, MRV; notably, thrombi are in unique locations including splanchnic and cerebral veins and even arteries | Receipt of adenovirus vaccine in previous 42 d | Obesity | High-dose corticosteroids |
| CBC, fibrinogen level, D-dimer, anti-PF4 IgG | Thrombosis and thrombocytopenia | Exogeneous estrogen replacement | IVIG 1 g/kg for 2 d | |||
| Elevated D-dimer | Women between the ages 18 and 50 y | Anticoagulation (avoidance of heparin if VITT suspected) | ||||
| Anti-PF4 antibodies | Platelet transfusion should be avoided |
CBC, Complete blood cell count; CT, computed tomography; MRV, magnetic resonance venography; PF4, platelet factor 4.
The administration of adenovirus vector–based vaccines was briefly paused in both Europe and the United States when there were increasing reports of VITT.91 After thorough evaluation, the administration of these vaccines was deemed safe; however, there now exists a warning, not a contraindication, for females between the ages of 18 and 50 years for the slightly increased risk for the development of VITT.92 However, a contraindication does exist for further viral vector COVID vaccines in those who have had VITT secondary to viral vector COVID-19 vaccination.93 In addition, because of the wide availability and superior efficacy of mRNA vaccines combined with the high mortality of this event, the CDC has recommended the use of Moderna or Pfizer vaccine over the J&J vaccine.94 In individuals who have experienced VITT, further administration of viral vector COVID-19 vaccines is contraindicated,95 and, in the setting of universal boosters, these individuals may receive mRNA vaccines as an acceptable alternative. It is important to note that VITT is a diagnosis of exclusion, because the existence of anti–platelet factor 4 antibodies occurs in other circumstances (eg, after cardiovascular surgery) and does not confirm the diagnosis of VITT.87 It is important to have a high index of suspicion, because this diagnosis is associated with high morbidity and mortality—between 30% and 40%.
Lymphadenopathy
Regional lymphadenopathy (LAD) was reported as an adverse effect in the original trials of both the Moderna and Pfizer vaccines at rates higher than the placebo group: 1.1% (vs 0.6%) and 0.3% (vs <0.1%) in clinical trials, respectively.9 , 10 Real-world studies have replicated findings of LAD after COVID-19 mRNA vaccination, demonstrating that this finding was more significant in individuals receiving the vaccine than in unvaccinated individuals with a risk difference of 78 events per 100,000.6 Thankfully, this side effect is physically harmless, albeit possibly uncomfortable for the patient, and is thought to represent the individual’s protective immune response to vaccination.10 , 96 Most LAD is unilateral axillary LAD (ipsilateral to the vaccination), but reports of supraclavicular and lateral neck LAD have been prominent as well.97 Because of these unconventional locations, in some cases, the presence of LAD has placed a psychological and financial burden on patients, because individuals seek further evaluation, especially in patients with previous diagnoses of malignancy.96 , 97 In most cases, LAD resolved within 4 weeks of either first or subsequent doses of vaccination.97 Although previous recommendations from the Society of Breast Imaging favored receipt of COVID-19 vaccine either before or at least 4 to 6 weeks after mammogram, recent evidence has shifted these recommendations, and in otherwise healthy, low-risk individuals, the Society of Breast Imaging does not recommend delay in mammography screening.98 These recommendations were, in part, modified because of recent study that showed the potential for LAD to persist for several months and the low proportion of individuals who went on to biopsy (8% of 537 individuals) after imaging.99 In general, individuals with LAD should be followed clinically to resolution with further evaluation in the absence of resolution after 5 weeks, unless additional history would suggest a high-risk situation.96 , 97
Moving Forward to Booster Doses and Beyond
Fortunately, the COVID-19 vaccines have been overwhelmingly safe and there are few reasons to exempt first or subsequent doses of the COVID-19 vaccines (Figure 2 ). Even with an immediate systemic reaction to the first dose, increasing evidence supports either tolerance of the same vaccine or tolerance of an alternate vaccine. With increasing experience, we know that most immediate reactions are unlikely to be IgE-mediated, and the vast majority of individuals have gone on to safely tolerate subsequent doses. Largely, the response to the adverse outcomes described above has been the question of how to safely move forward with widespread immunization. In an interim analysis of mRNA COVID-19 vaccines, the incidence of specific serious outcomes was not higher 1 to 21 days postvaccination compared with 22 to 42 days postvaccination.20 In addition, fortunately, and contrary to much anxiety in the general population about serious vaccine adverse events appearing years later, past experience has told us that vaccine-related adverse reactions are unlikely to occur more than 2 months following initial vaccination.6 , 22 , 100 Reassuringly, the reactions described—all of which have occurred in the first few weeks following vaccination—are overall rare (Table I). Thankfully, current data and the availability of different types of COVID-19 vaccines have reassured us that it would be an exceptionally rare circumstance that an adverse reaction to one COVID-19 vaccine precludes administration of any other, or even the same, COVID-19 vaccine with allergist guidance. Our knowledge of this concept is increasing, which is extremely important not only for the COVID-19 vaccines but also for many other pathogens and diseases of global importance to follow that will benefit from mRNA therapeutics. Individuals with an existing IgE-mediated PEG allergy were previously advised against receiving mRNA vaccines due to the PEG2000 lipid nanoparticle carrier; however, we now know that this may be tolerated as in patients with PEG-asparaginase hypersensitivity,39 , 40 and, more recently, known PEG allergy.38 , 41 As discussed, most of the individuals with documented first-dose immediate reactions went on to tolerate the second dose without difficulty. These individuals did not have elevated tryptase when measured, and most had no reactions after the second dose.23 , 24 As we learn more about immediate reactions to COVID-19 vaccines, the evidence suggests that allergist evaluation is likely to further guide receipt of routine vaccination in observed, low-acuity settings, without the need for skin testing, in the large majority of cases.
Figure 2.
Guidance for exemptions. Examples of questions that may be asked of an allergist/immunologist in the evaluation of an individual with concerns about COVID-19 vaccination accompanied by proposed advice and solutions.
CDC recommendations as of April 2022 for individuals experiencing mRNA-associated myocarditis/pericarditis include a precaution to avoid subsequent doses of any COVID-19 vaccine without an absolute contraindication.93 If a patient reports an episode of thrombosis with thrombocytopenia syndrome, the CDC currently advises that further adenovirus-based COVID-19 vaccines are contraindicated, especially in light of the efficacy and availability of mRNA vaccines, which are safe for these individuals.93 Many patients report a history of non–IgE-mediated reactions after receipt of previous vaccines. Nonspecific, delayed symptoms are unlikely to be related to IgE toward vaccine components and is not predictive of future reactions to vaccines, so these individuals may proceed to routine vaccination without allergy consultation needed. With more than 1 year of data, there is now a better idea of the rates of COVID-19–associated infection and mortality among those vaccinated versus those who are unvaccinated. The CDC reports the average weekly incidence rate of COVID-19 infection from July to November 2021 for those unvaccinated to be 3.1 times higher than for those vaccinated (with any vaccine) for COVID-19; during the same time period, the average weekly incidence rate of COVID-19–associated deaths was 16.3 times higher in those unvaccinated when compared with those vaccinated.101 The protection, especially against death from COVID-19 infection, afforded by vaccination outweighs the potential conferred risks for most, if not all, candidates.
As preventive efforts for COVID-19 infection have evolved, additional oral and injectable medications have been approved for emergency use authorization by the Food and Drug Administration. Specifically, Evusheld (tixagevimab copackaged with cilgavimab), a long-acting, combination mAb against the SARS-CoV-2 spike protein that is active against the Omicron variant, is approved for preexposure prophylaxis of COVID-19 in individuals with an underlying immunocompromised state or who may be unable to receive any of the COVID-19 vaccines.102 , 103 With initial approval, there have been concerns about the supply of these antibodies and availability especially in resource-poor settings. In light of the growing body of information supporting routine vaccination, including in those with immediate or delayed hypersensitivity history, it is likely that the pool of individuals with histories of suspected COVID-19 vaccine hypersensitivity reactions who may require Evusheld will be minimal.
Conclusions
If we come back to our case, we appreciate the example of an individual who had an immediate reaction to the first dose of an mRNA vaccine and subsequently tolerated the second dose safely. As highlighted in this review, although initially thought to be insightful, we now know that skin testing is not necessary in such a patient moving forward. This vaccine tolerance of the second dose points away from an IgE-mediated mechanism of her reaction. Recent case series have demonstrated tolerance of mRNA vaccines in patients with a confirmed or highly suspected history of PEG allergy. These examples are reassuring and suggest that, as we accumulate more data, the initial abundance of caution that led us to risk stratify patients on the basis of knowledge of a previous reaction to a “component of the mRNA vaccines” continues to shift toward the vast majority of all patients being either outright eligible for vaccination or referred to the allergist for “vaccine allergy delabeling.”
In this mature phase of the pandemic, we can be reassured that the growing body of data supports the continued safe and efficacious administration of COVID-19 vaccines, though there still exist many unanswered questions (Table IV ). It is important for providers to become familiar with the presenting features of the major vaccine reactions and take the appropriate steps for further evaluation and treatment in the rare circumstances when it is needed. Immediate and cutaneous reactions, along with myopericarditis, have no long-term sequelae that have been identified. Individuals with immediate and benign, delayed cutaneous reactions have additionally gone on to safely tolerate further doses in the vaccine series after recovering from the acute adverse events, oftentimes without the side effects experienced after the first dose. VITT/thrombosis with thrombocytopenia syndrome is rarer than these events but is associated with increased morbidity and mortality when compared with immediate, cutaneous, and myocardial side effects, and the age and medical history of the individual seeking vaccination should be taken into consideration. It is evident, with current data, that COVID-19 vaccination poses a much lower risk of adverse events than infection with SARS-CoV-2. In addition, even in the face of continued emergence of variants, completion of COVID-19 vaccination, including booster doses, provides the maximum protection against severe illness and hospitalization, which is particularly important for those with medical comorbidities and other high-risk individuals, because implementation of immunization on widespread levels globally has been life-saving for these individuals.
Table IV.
Knowns and unknowns—Examples of questions that have been answered and those that remain unanswered at this phase of the pandemic and vaccine rollout
| Adverse reaction | What we don’t know | What we do know |
|---|---|---|
| Immediate systemic reactions |
|
|
| Delayed cutaneous and other hypersensitivity reactions |
|
|
| Myocarditis/pericarditis |
|
|
| Thrombosis with thrombocytopenia syndrome |
|
|
SCAR, Severe cutaneous adverse reaction.
Footnotes
E.J.P. reports grants from the National Institutes of Health (grant nos. P50GM115305, R01HG010863, R01AI152183, R21AI139021, and U01AI154659) and from the National Health and Medical Research Council of Australia. Funders played no role in any aspect of this review.
Conflicts of interest: E. J. Phillips reports grants from the National Institutes of Health (grant nos. P50GM115305, R01HG010863, R01AI152183, R21AI139021, and U01AI154659) and from the National Health and Medical Research Council of Australia; reports royalties from UpToDate and consulting fees from Janssen, Vertex, Biocryst, and Regeneron; is codirector of IIID Pty Ltd, which holds a patent for HLA- B∗57:01 testing for abacavir hypersensitivity; and has a patent pending for Detection of Human Leukocyte Antigen-A∗32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
The case described in this review is a representative patient based on experiences in our outpatient clinic and does not correspond to an actual patient.
References
- 1.Randall T., Sam C., Tartar A., Murray P., Cannon C. More than 10.1 billion shots given: Covid-19 Tracker 2022. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ Updated April 25, 2022. Accessed April 25, 2022.
- 2.ClinicalTrials.gov A phase 1 study to evaluate the safety and immunogenicity of eOD-GT8 60mer mRNA vaccine (mRNA-1644) and core-g28v2 60mer mRNA vaccine (mRNA-1644v2-Core) 2021. https://clinicaltrials.gov/ct2/show/NCT05001373 Updated September 30, 2021. Accessed October 29, 2021.
- 3.ClinicalTrials.gov Safety, tolerability, and immunogenicity of Zika vaccine mRNA-1893 in healthy flavivirus seropositive and seronegative adults. 2021. https://clinicaltrials.gov/ct2/show/NCT04064905 Updated April 22, 2021. Accessed October 29, 2021.
- 4.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 6.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., Li T. Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: challenges and future perspectives. Pharm Res. 2021;38:473–478. doi: 10.1007/s11095-021-03015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer K.J., Lambe T., Rollier C.S., Spencer A.J., Hill A.V., Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Nogrady B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature. 2021;595:339–340. doi: 10.1038/d41586-021-01813-2. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 16.Team CC-R, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P., et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Drug Control and Prevention Selected adverse events reported after COVID-19 vaccination. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html Updated November 1, 2021. Accessed November 4, 2021.
- 20.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhawt M., Abrams E.M., Shaker M., Chu D.K., Khan D., Akin C., et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9:3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krantz M.S., Bruusgaard-Mouritsen M.A., Koo G., Phillips E.J., Stone C.A., Jr., Garvey L.H. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krantz M.S., Kwah J.H., Stone C.A., Jr., Phillips E.J., Ortega G., Banerji A., et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanijcharoenkarn K., Lee F.E., Martin L., Shih J., Sexton M.E., Kuruvilla M.E. Immediate reactions after the first dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA vaccines do not preclude second-dose administration. Clin Infect Dis. 2021;73:2108–2111. doi: 10.1093/cid/ciab448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alhumaid S., Al Mutair A., Al Alawi Z., Rabaan A.A., Tirupathi R., Alomari M.A., et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2021;17:109. doi: 10.1186/s13223-021-00613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2021.06.010. :3308-20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu D.K., Abrams E.M., Golden D.B.K., Blumenthal K.G., Wolfson A.R., Stone C.A., Jr., et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. 2022;182:376–385. doi: 10.1001/jamainternmed.2021.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risma K.A., Edwards K.M., Hummell D.S., Little F.F., Norton A.E., Stallings A., et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147 doi: 10.1016/j.jaci.2021.04.002. :2075-82.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips E.J. Allergic reactions after COVID-19 vaccination—putting risk into perspective. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22326. [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov COVID19 SARS vaccinations: systemic allergic reactions to SARS-CoV-2 vaccinations (SARS) 2021. https://clinicaltrials.gov/ct2/show/NCT04761822 Updated September 1, 2021. Accessed November 13, 2021.
- 33.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habran M., Vandebotermet M., Schrijvers R. Polyethylene glycol allergy and immediate-type hypersensitivity reaction to COVID-19 vaccination: case report. J Investig Allergol Clin Immunol. 2022;32:234–235. doi: 10.18176/jiaci.0740. [DOI] [PubMed] [Google Scholar]
- 36.Sellaturay P., Nasser S., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ieven T., Van Weyenbergh T., Vandebotermet M., Devolder D., Breynaert C., Schrijvers R. Tolerability of polysorbate 80 containing COVID-19 vaccines in confirmed PEG allergic patients. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2021.09.039. :4470-2.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brockow K., Mathes S., Fischer J., Volc S., Darsow U., Eberlein B., et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. 2022;77:2200–2210. doi: 10.1111/all.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo G., Anvari S., Friedman D.L., Zarnegar-Lumley S., Szafron V., Kahwash B.M., et al. mRNA COVID-19 vaccine safety in patients with previous immediate hypersensitivity to pegaspargase. J Allergy Clin Immunol Pract. 2022;10:322–325. doi: 10.1016/j.jaip.2021.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mark C., Gupta S., Punnett A., Upton J., Orkin J., Atkinson A., et al. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard M., Drolet J.P., Masse M.S., Filion C.A., ALMuhizi F., Fein M., et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. 2022;10 doi: 10.1016/j.jaip.2021.11.021. :620-5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuong L.C., Capucilli P., Staicu M., Ramsey A., Walsh E.E., Shahzad Mustafa S. Graded administration of second dose of Moderna and Pfizer-BioNTech COVID-19 mRNA vaccines in patients with hypersensitivity to first dose. Open Forum Infect Dis. 2021;8:ofab507. doi: 10.1093/ofid/ofab507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cahill J.A., Kan M. Successful administration of second dose of BNT162b2 COVID-19 vaccine in two patients with potential anaphylaxis to first dose. Allergy. 2022;77:337–338. doi: 10.1111/all.14996. [DOI] [PubMed] [Google Scholar]
- 44.Kozma G.T., Shimizu T., Ishida T., Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-5:163–175. doi: 10.1016/j.addr.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 46.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7 doi: 10.1016/j.jaip.2018.12.003. :1533-40.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon D.E., Kovarik C.L., Damsky W., Rosenbach M., Lipoff J.B., Tyagi A., et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113–121. doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson L.B., Fu X., Hashimoto D., Wickner P., Shenoy E.S., Landman A.B., et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021;157:1000–1002. doi: 10.1001/jamadermatol.2021.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ackerman M., Henry D., Finon A., Binois R., Esteve E. Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35:e423–e425. doi: 10.1111/jdv.17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Q., Fathy R., McMahon D.E., Freeman E.E. COVID-19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653–673. doi: 10.1016/j.det.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dash S., Sirka C.S., Mishra S., Viswan P. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615–1617. doi: 10.1111/ced.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elboraey M.O., Essa E. Stevens-Johnson syndrome post second dose of Pfizer COVID-19 vaccine: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:e139–e142. doi: 10.1016/j.oooo.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang S.Y., Park S.Y., Kim J.H., Lee S.M., Lee S.P. COVID-19 vaccine-induced acute generalized exanthematous pustulosis. Korean J Intern Med. 2021;36:1537–1538. doi: 10.3904/kjim.2021.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lospinoso K., Nichols C.S., Malachowski S.J., Mochel M.C., Nutan F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine. JAAD Case Rep. 2021;13:134–137. doi: 10.1016/j.jdcr.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agaronov A., Makdesi C., Hall C.S. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96–97. doi: 10.1016/j.jdcr.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157:716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez-Nieto D., Hammerle J., Fernandez-Escribano M., Moreno-Del Real C.M., Garcia-Abellas P., Carretero-Barrio I., et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35:e425–e427. doi: 10.1111/jdv.17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dreskin S.C., Halsey N.A., Kelso J.M., Wood R.A., Hummell D.S., Edwards K.M., et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. doi: 10.1186/s40413-016-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianchi L., Biondi F., Hansel K., Murgia N., Tramontana M., Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: limits of intradermal testing. Allergy. 2021;76:2605–2607. doi: 10.1111/all.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birabaharan M., Kaelber D.C., Karris M.Y. Risk of herpes zoster reactivation after messenger RNA COVID-19 vaccination: a cohort study. J Am Acad Dermatol. Published online November 23, 2021. [DOI] [PMC free article] [PubMed]
- 63.Bostan E., Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 64.Eid E., Abdullah L., Kurban M., Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021;93:5231–5232. doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tessas I., Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35:e620–e622. doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vastarella M., Picone V., Martora F., Fabbrocini G. Herpes zoster after ChAdOx1 nCoV-19 vaccine: a case series. J Eur Acad Dermatol Venereol. 2021;35:e845–e846. doi: 10.1111/jdv.17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walter R., Hartmann K., Fleisch F., Reinhart W.H., Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet. 1999;353:810. doi: 10.1016/S0140-6736(99)00623-6. [DOI] [PubMed] [Google Scholar]
- 68.Munavalli G.G., Knutsen-Larson S., Lupo M.P., Geronemus R.G. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63–68. doi: 10.1016/j.jdcr.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 73.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shay D.K., Shimabukuro T.T., DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021;6:1115–1117. doi: 10.1001/jamacardio.2021.2821. [DOI] [PubMed] [Google Scholar]
- 75.Husby A., Hansen J.V., Fosbol E., Thiesson E.M., Madsen M., Thomsen R.W., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buchan S.A., Seo C.Y., Johnson C., Alley S., Kwong J.C., Nasreen S., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. Preprint published online December 5, 2021. [DOI] [PMC free article] [PubMed]
- 77.Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Umei T.C., Kishino Y., Watanabe K., Shiraishi Y., Inohara T., Yuasa S., et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4:350–352. doi: 10.1016/j.cjco.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung M.K., Zidar D.A., Bristow M.R., Cameron S.J., Chan T., Harding C.V., III, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tobaiqy M., MacLure K., Elkout H., Stewart D. Thrombotic adverse events reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and clinical outcomes in the EudraVigilance database. Vaccines (Basel) 2021;9:1326. doi: 10.3390/vaccines9111326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliver S.E., Wallace M., See I., Mbaeyi S., Godfrey M., Hadler S.C., et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCrae K.R. Thrombotic thrombocytopenia due to SARS-CoV-2 vaccination. Cleve Clin J Med. Published online May 6, 2021. [DOI] [PubMed]
- 88.Sharifian-Dorche M., Bahmanyar M., Sharifian-Dorche A., Mohammadi P., Nomovi M., Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination: a systematic review. J Neurol Sci. 2021;428:117607. doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franchini M., Liumbruno G.M., Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021;107:173–180. doi: 10.1111/ejh.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bussel J.B., Connors J.M., Cines D.B., Dunbar C.E., Michaelis L.C., Kreuziger L.B., et al. Thrombosis with thrombocytopenia syndrome (also termed vaccine-induced thrombotic thrombocytopenia) 2021. https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia Updated August 12, 2021. Accessed November 4, 2021.
- 91.Sadoff J., Davis K., Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination—response from the manufacturer. N Engl J Med. 2021;384:1965–1966. doi: 10.1056/NEJMc2106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacNeil J.R., Su J.R., Broder K.R., Guh A.Y., Gargano J.W., Wallace M., et al. Updated recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html Updated March 30, 2022. Accessed March 30, 2022.
- 94.Centers for Disease Control and Prevention CDC endorses ACIP’s updated COVID-19 vaccine recommendations: media statement. 2021. https://www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html Updated December 16, 2021. Accessed February 13, 2022.
- 95.Centers for Disease Control and Prevention Janssen COVID-19 vaccine (Johnson & Johnson) https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/index.html#:∼:text=Contraindications%3A,a%20component%20of%20the%20vaccine Updated February 15, 2022. Accessed February 16, 2022.
- 96.Tu W., Gierada D.S., Joe B.N. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imaging Cancer. 2021;3 doi: 10.1148/rycan.2021210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keshavarz P., Yazdanpanah F., Rafiee F., Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolfson S., Kim E., Plaunova A., Bukhman R., Sarmiento R.D., Samreen N., et al. Axillary adenopathy after COVID-19 vaccine: no reason to delay screening mammogram. Radiology. 2022;303:297–299. doi: 10.1148/radiol.213227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grimm L., Srini A., Dontchos B., Daly C., Tuite C., Sonnenblick E., et al. Revised SBI recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. 2022. https://www.sbi-online.org/Portals/0/Position%20Statements/2022/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination_updatedFeb2022.pdf Updated February 2022. Accessed March 30, 2022.
- 100.Stone C.A., Jr., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson A.G., Amin A.B., Ali A.R., Hoots B., Cadwell B.L., Arora S., et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA. 2022;327:384–385. doi: 10.1001/jama.2021.24931. [DOI] [PubMed] [Google Scholar]
- 103.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes new long-acting monoclonal antibodies for pre-exposure prevention of COVID-19 in certain individuals. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure Updated December 8, 2021. Accessed February 14, 2022.