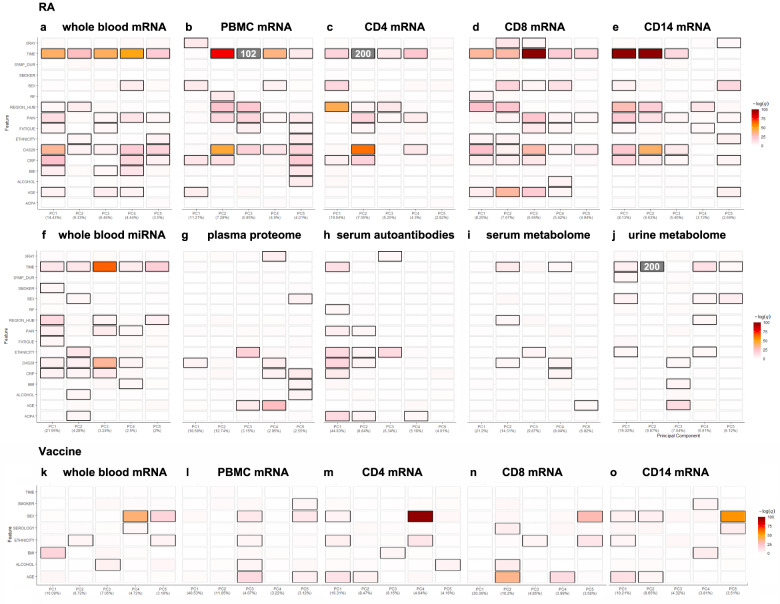
Fig. 4.
Unsupervised PCA Driver analysis of multi-omic compartments across TACERA early RA patients and Hepatitis B vaccine recipients, showing clinical features and their degree of association with Principal Components (PC) 1–5, percentage indicating variation accounted for by the PC and with coloring indicating the –log q-value of the association (scaled to maximum range of PC1 across all compartments, off scale associations are indicated in grey with –log q written). Significant drivers with an FDR threshold of 5% are indicated by outline. Specific drivers of variation in expression in analysed samples are indicated in TACERA patients in (a) Whole Blood mRNA, (b) PBMC mRNA, (c) CD4 mRNA, (d) CD8 mRNA, (e) CD14 mRNA, (f) Whole Blood micro RNA, (g) Plasma proteomics, (h) Serum autoantibodies, (i) Serum metabolome, (j) Urine metabolome. In VACCINE recipients in (k) Whole Blood mRNA, (l) PBMC mRNA, (m) CD4 mRNA, (n) CD8 mRNA, (o) CD14 mRNA. Clinical features include XRAY (quantitative measure of bone erosion at sample timepoint (0 or 6 m)), TIME (sample annotation at baseline or 6-months), SYMP_DUR (Symptom duration at diagnosis), SMOKER (smoking status Y, N, Previous), SEX (M/F), RF (Rheumatoid factor positive Y/N), PAIN (quantitative measure of pain at sample timepoint (0 or 6 m)), FATIGUE (quantitative measure of fatigue at sample timepoint (0 or 6 m)), ETHNICITY (Ethnic origin), DAS28 (disease activity score in 28 joints at sample timepoint (0 or 6 m)), CRP (quantitative measure of C-reactive protein at sample timepoint (0 or 6 m)), BMI (Body Mass Index at baseline), ALCOHOL (Y/N), AGE (Age at baseline), ACPA (anti-citrullinated protein antibody positive Y/N), SEROLOGY (Hepatitis B serology at week 9). In the TACERA cohort in 3a-j the first two PCs are closely associated with DAS28 scores in Whole Blood, PBMC mRNA, CD4 mRNA, and CD14 mRNA; and rather less closely associated with the other platforms. In 3k-o, in contrast to the highly dysregulated immune system seen in RA patients, the biological perturbation following a vaccine-related immune challenge in the healthy volunteers appears negligible.