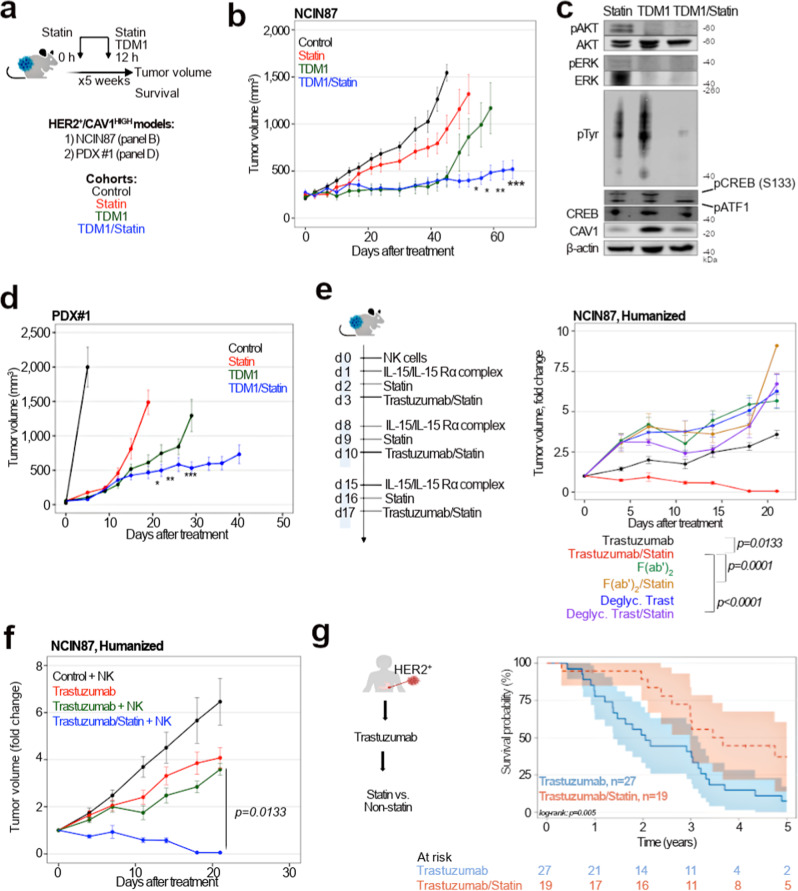
Fig. 4. Lovastatin enhances TDM1 efficacy and Trastuzumab-mediated ADCC.
a–d Superior in vivo therapeutic efficacy of TDM1 combined with lovastatin when compared with TDM1 alone. a Intravenous TDM1 administration 5 mg/kg weekly (for 5 weeks) was started at day 0. Lovastatin (4.15 mg/kg of mice) was orally administrated 12 h prior to and simultaneously with the intravenous injection of TDM1. Lovastatin enhanced TDM1 efficacy of nu/nu female mice bearing NCIN87 gastric xenografts (b), and NSG mice bearing CAV1-high PDXs (d). *P < 0.05, **P < 0.01, ***P < 0.001 based on a Student’s t test (n = 8–10 mice per group). c Western blot analyses of AKT, ERK, Tyr, CAV1, and CREB protein expression and phosphorylation in NCIN87 xenografts at 40 days after treatment with lovastatin, TDM1, or TDM1/lovastatin. e NSG mice bearing NCIN87 xenografts were intravenously injected 1 × 106 human NK cells at day 0. One day after NK cells tail vein injection, the IL-15/IL-15Rα complex was intraperitoneally administered at a dose of 1.25 μg/mouse. Trastuzumab or Trastuzumab/lovastatin efficacy was then evaluated during a cytokine-dependent NK expansion phase (week 1–week 3). Lovastatin enhanced Trastuzumab efficacy in NSG mice humanized with NK cells and bearing NCIN87 xenografts (n = 8–10 mice per group, mean ± S.E.M.). Statistical analyses performed using ANOVA coupled to Scheffé's method. f Trastuzumab/lovastatin efficacy is higher than the combination of Fc-silent Trastuzumab (Trastuzumab F(ab’)2 fragments or deglycosylated Trastuzumab) in NSG mice humanized with NK cells and bearing NCIN87 xenografts (n = 8–10 mice per group, mean ± S.E.M.). Statistical analyses performed using ANOVA coupled to Scheffé's method. g Kaplan–Meier analysis of statin use and HER2-expressing GC disease outcome in patients treated with Trastuzumab. Patients without statin treatment (blue color, n = 27) have a worse survival than patients treated with statin (red color, n = 19). Log rank; p = 0.005. Source data are provided as a Source Data file.