Abstract
Purpose
Placement of dental implants has evolved to be an advantageous treatment option for rehabilitation of the fully or partially edentulous mandible. In case of extensive horizontal bone resorption, the bone volume needs to be augmented prior to or during implant placement in order to obtain dental rehabilitation and maximize implant survival and success.
Methods
Our aim was to systematically review the available data on lateral augmentation techniques in the horizontally compromised mandible considering all grafting protocols using xenogeneic, synthetic, or allogeneic material. A computerized and manual literature search was performed for clinical studies (published January 1995 to March 2021).
Results
Eight studies ultimately met the inclusion criteria comprising a total of 276 procedures of xenogeneic, allogeneic, or autogenous bone graft applications in horizontal ridge defects. Particulate materials as well as bone blocks were used as grafts with a mean follow-up of 26.0 months across all included studies. Outcome measures, approaches and materials varied from study to study. A gain of horizontal bone width of the mandible with a mean of 4.8 mm was observed in seven of eight studies. All but one study, reported low bone graft failure rates of 4.4% in average.
Conclusions
Only limited data are available on the impact of different horizontal augmentation strategies in the mandible. The results show outcomes for xenogeneic as well as autologous bone materials for horizontal ridge augmentation of the lower jaw. The use of allogeneic bone-block grafts in combination with resorbable barrier membranes must be re-evaluated. Randomized controlled clinical trials are largely missing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40729-022-00421-7.
Keywords: Augmentation, Horizontal, Lateral, Mandible, Lower jaw
Background
Dental implantology has evolved as an advantageous treatment method for dental rehabilitation and prosthetic restoration of partial or fully edentulous jaws [1, 2]. Sufficient amount and quality of bone remains a determining requirement for successful long-time implant survival and success and to prevent peri-implant disease [3–5]. Physiologic bone loss has been reported in various studies as a logical consequence after tooth extraction [6, 7]. In cases of severe bone loss—mostly due to long time interval since tooth removal, unfavorable load, infection, trauma, or other reasons—bone frequently needs to be augmented prior to or during implant placement [8, 9]. Different augmentation techniques have been developed depending on localization, extent, and configuration of the bone defect [10, 11].
If alveolar bone loss is limited, bone splitting and spreading are useful techniques for adaptation to the local conditions, although this may be difficult to apply in strong cortical bone such as the mandible [12]. Narrow diameter implants have also been proven to serve as possible solutions in horizontally compromised bone [13]. However, in cases of severe horizontal bone loss, horizontal augmentation of the mandibular alveolar ridge may be necessary and can be achieved by a variety of surgical approaches [9]. These procedures include insertion of bone grafts or stimulating bone formation in terms of guided bone regeneration [14–17]. Autologous bone is still regarded as gold standard given its biological properties [9, 18]. However, due to aspects like donor site morbidity, prolonged time of surgery and unpredictable resorption dynamics, a variety of allografts, xenografts and synthetic materials have been introduced into the market and numerous compositions are commercially available. Moreover, these grafts are commonly combined with autologous bone to improve compatibility and combine the advantages of these materials [19]. Additionally, this diversity is extended by different surgical techniques and protocols. Due to the enormous number of materials and techniques, a randomized controlled trial to evaluate and compare all the different approaches would be impossible to conduct. However, there exist scientific reports on the topic that can provide a considerable body of knowledge. For instance, Troeltzsch et al. provide a comprehensive analysis of the available literature for the augmentation of the alveolar ridge [20]. Bone formation in the augmented areas varied from 33.2 ± 14.9% for allogeneic grafts to 56.0 ± 25.6% for mixtures of autogenous and other grafting materials. The authors derive a horizontal gain of 3.7 mm for particulate, compared to 4.5 mm for block grafts. Despite a detailed evaluation of horizontal and vertical dimension, analysis of the donor site (autogenous iliac crest, calvarium, mandible, allogeneic) or the subclassification of the results with regard to the use of membranes and meshes, distinct results for the horizontally compromised lower jaw as recipient site remain elusive.
Furthermore, implant survival and success in concomitantly vs. subsequently placed dental implants are not reported uniformly, so that this aspect remains unclear to date. Thus, the aim of this report was to systematically review the available data and potentially draw conclusions about the efficacy in gaining bone width, implant survival and success rates after or accompanying horizontal ridge augmentation procedures using autologous, xenogeneic, synthetic, or allogeneic materials or combinations of these in cases of bone loss in the lower jaw, which require lateral augmentation. The idea was to systematize approaches and give recommendations on this complex and relevant subject by assessing the efficacy of grafting materials with respect to clinically relevant parameters.
Results
Study characteristics
The initial electronic search identified a total of 15,643 titles (Fig. 1). 890 studies were selected for further assessment of the abstract after screening of the titles. 64 articles were added by manual search. Out of these, a total of 866 were excluded. Subsequently, 88 full-text articles were obtained, and “Materials and methods” and “Results” sections were investigated. Only eight studies ultimately met the inclusion criteria (Fig. 1). The reasons for exclusion are depicted in Table 1.
Fig. 1.
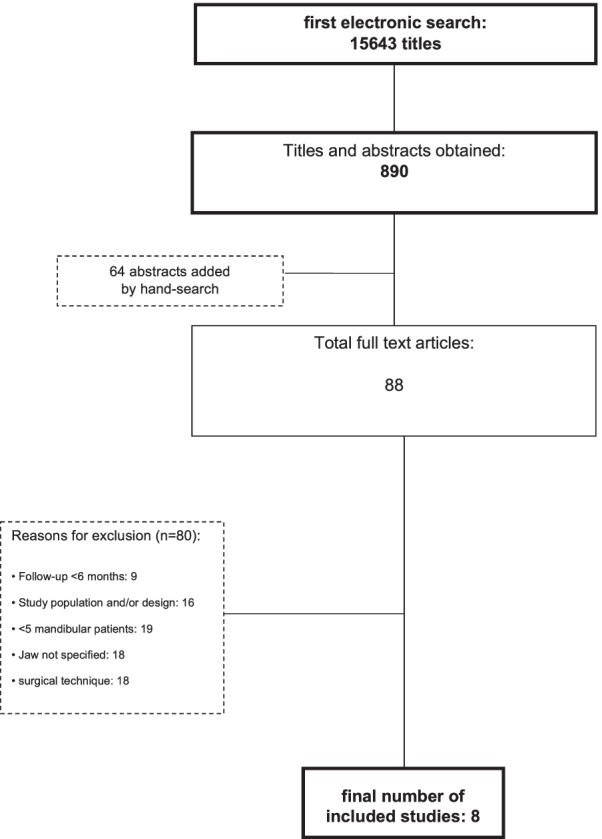
Tree diagram of exclusion/inclusion of studies for this systematic review
Table 1.
Specification of excluded studies after full-text analysis
Data synthesis and/or meta-analysis were not performed due to the heterogeneity of the study designs and parameters. Risk of bias of each study is given in Table 2. Information on additional risk of bias across studies is elaborated in “Discussion” section.
Table 2.
Assessment of risk of bias according to The Cochrane Collaboration’s tool for RCTs, the ROBINS-I tool was applied for prospective cohorts, and the Checklist for Case Series from the Joanna Briggs Institute
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Amorfini et al. | + | + | + | + | + | + | + |
| Nissan et al. | ? | ? | ? | ? | + | + | + |
| Barbu et al. | ? | ? | ? | + | + | + | + |
| Beitlitum et al. | − | − | − | − | + | + | + |
| Di Stefano et. al. | − | − | − | − | + | + | + |
| Schwartz-Arad et al. | − | − | − | − | + | + | + |
| Silva et al. | − | − | − | − | + | + | + |
| Urban et al. | − | − | − | − | + | + | + |
+: low, −: high, and ?: unclear risk of bias
Efficacy of augmentation procedures in horizontally resorbed ridges
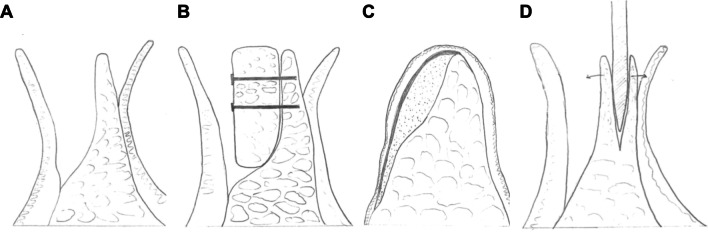
Eight studies met the inclusion criteria containing a total of 276 procedures of xenogeneic, allogeneic, or autogenous bone graft applications in horizontal ridge defects. Outcome measures, approaches and materials varied from study to study. The mean follow-up was 26.0 months across all included studies, with a maximum mean observation period of 40.5 months, and minimum mean observation period of 6 months. Particulate material as well as bone blocks were used as grafts in the studies (see Figs. 2 and 3).
Fig. 2.
Schematic of horizontal bone loss in the lower jaw after crestal gingival exposure (A). Principle of bone-block grafting and fixation with screws (B). Depiction of lateral augmentation using particulate bone and membrane placement for coverage (C). Gaining of horizontal bone width by surgical splitting of the alveolar ridge (D)
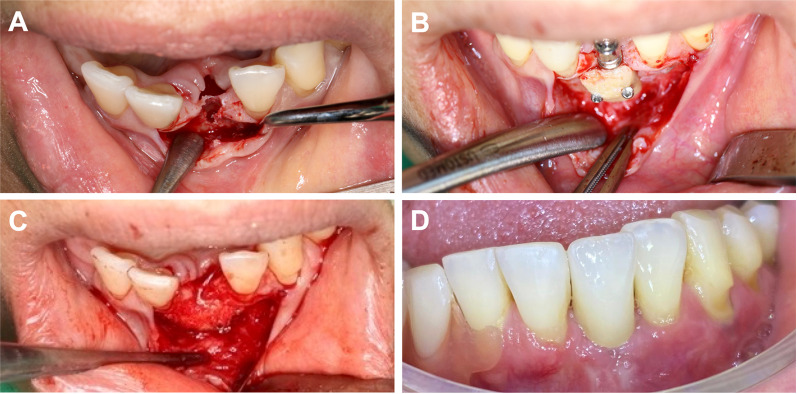
Fig. 3.
Clinical example of a 41-year-old female patient presenting with missing tooth 31 and consecutive horizontal bone loss (A). For dental, functional and esthetic rehabilitation, simultaneous implant placement and lateral augmentation was performed. Implantation of Conelog Progressive Line, harvesting of a retromolar bone block from the mandibular angle with a trephine drill, augmentation and microscrew fixation (1.0-mm steel screws) after rounding of the edges with a burr (B). Relining and fitting with particulate bone placement (C). After 5 months of healing time, implant exposure and fixation screw removal, sequential abutment fitting and placement of dental crown were performed (D)
The autologous block grafts used in the included studies were taken from the mandibular ramus [99, 100] or as bone chips from the posterior mandible/retromolar region [100–102]. A combination of autografts with xenografts or allografts was used in four studies. All augmentation sites were covered, either by membranes or platelet-poor plasma. Table 3 shows the variety of outcome measures used in the included studies.
Table 3.
Methodological characteristics of the selected studies, the regenerative objective (simultaneous or staged), the types of interventions and measured outcomes
| Author | Year | Study design | Setting | Type of augmentation | Regenerative objective | Interventions | Test/control, n | Preparation | Outcomes measures | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Control | Patients | Sites | ||||||||
| Amorfini et al. [101] | 2014 | RCT, parallel, allocation ratio of 1:1, split-mouth model | Private practice | Lateral | Accompanying implant placement | Corticocancellous allograft (iliac crest) + collagen membrane (Biogide®, Geistlich AG) + rhPDGF-BB (GEM 21S®, Osteohealth) or saline solution | Autologous bone chips + bovine bone xenograft (BioOss®, Geistlich) + collagen membrane (Bio-Gide®, Geistlich) + rhPDGF-BB (GEM 21S® Osteohealth) or saline solution | 8/8 | 8/8 | Block graft (treatment) and particulate material (control) | BV, RA, BLCT, BOP, PPD, PI, mBL |
| Barbu et al. [100] | 2016 | RCT | University clinic | Lateral | Two-staged | Autogenous ramus block (retromolar) + Autogenous particulate bone + PRF membrane (Group 1) | Autogenous ramus block (retromolar) + Autogenous particulate bone chips mixed with xenograft particulate bone + Pericardium membrane (Group 2) | 12/12 | 12/12 | Crestal incision, block graft, cortical perforation of recipient bed | RA, IS, BG, EX, PGF, TGF |
| Beitlitum et al. [117] | 2018 | Retrospective study | Two periodontal practices, one university dental clinic | Lateral | Two-staged | Particulate mineralized bone allograft (MinerOss, BioHorizons or Maxgraft, Botiss) covered with a cross-linked resorbable collagen barrier membrane | – | 15/– | 16/– | Crestal incision, particulate allograft | RA, EX, IS, ME, PGF, TGF |
| Di Stefano et al. [104] | 2009 | Case series | Private practice | Lateral | Two-staged | Onlay graft of equine spongy bone layer (Osteoplant Flex, Bioteck, Italy) + titanium-reinforced membrane | – | 5/5 | 5/5 | Onlay xenograft | HA, PGF, TGF, RA |
| Nissan et al. [103] | 2011 | RCT | – | Lateral and vertical | Two-staged | Cancellous allograft + mineralized freeze-dried bone allograft (OraGraft, Lifenet), or bovine bone xenograft (BioOss®, Geistlich) + Membranes: Ossix Plus, OraPharma; Ossix, Biomet; Bio-Gide, Geistlich; | Pre-augmentation measurements of crest width and height | 21/– | 29/– | Crestal incision, block graft | WG, WR, ME, HR |
| Schwartz-Arad et al. [99] | 2016 | Retrospective study | Outpatient surgical center | Lateral and vertical | Two-staged | Autologous intraoral graft + bovine bone xenograft (BioOss®, Geistlich AG) + platelet-rich plasma (PRP) or platelet-poor plasma (PPP) | – | n/a | 115/– | Crestal incision, block graft | RA, EX, EXH, EXW, Inf, IS, ISC, ME, PGF, TGF, SP |
| Silva et al. [105] | 2017 | Prospective study | University clinic | Lateral and vertical | Two-staged | Fresh-frozen bone-block allograft (femoral epiphyses) from tissue bank (UNIOSS, Brazil) + BBM granules (BioOss, Geistlich, Switzerland) + resorbable collagen membrane (Bio-Gide, Geistlich, Switzerland) | – | 20/– | 50/– | block allograft, particulate material | RA, EX, PGF, TGF, BV, HA, IS, mBL, SP, WG |
| Urban et al. [102] | 2013 | Case series | University Clinic | Lateral | Two-staged | xenograft (inorganic bovine bone-derived mineral, Bio-Oss) + autologous bone graft + resorbable collagen membrane | – | 13/– | 16/– | particulate material | BV, IS, ISC, PGF, SP, WG, WR |
BOP bleeding on probing, BV bone volume changes, CAL peri-implant clinical attachment level, CCT controlled clinical trial, ePTFE expanded polytetrafluoroethylene, EX exposure, EXH exposed site height, EXW exposed site width, HA histological assessment, IS implant survival, mBL mean marginal bone loss, ME membrane exposure, PGF partial graft failure, PI peri-implant plaque index, PPD peri-implant probing depth, PRF platelet-rich fibrin, RA radiographic assessment, POP penetration of the probe, RCT randomized controlled trial, rh-BMP2 recombinant human bone morphogenic protein 2, rhPDGF-BB recombinant human platelet-derived growth factor-BB, SP success rate procedure, TGF total graft failure, WG width gain, WR width reduction
Even though this review aims to report the results in a standardized way, the wide range of different settings, study protocols and outcome parameters limit conclusions in an evidence-based manner. Hence, only circumscribed quantitative data could be analyzed and this review should be perceived with a narrative approach. The study design ranged from (randomized) controlled clinical trials to pro- and retrospective studies. Particularly, the outcome measures varied extensively in the presented reports. All studies referred to clinical assessment and radiographic imaging when reporting outcome parameters (Table 4).
Table 4.
Assessment parameters and study results
| Author | Assessment method | Initial horizontal width in mm | Final horizontal width in mm | Horizontal gain in mm | Loss in mm | Bone formation in % | bone graft failure in % | Implant survival in % at last follow-up |
|---|---|---|---|---|---|---|---|---|
| Amorfini et al. [101] | Clinical assessment; CBCT scan | – | – | 5.7 | 0.2 | – | 0 | 100 |
| Barbu et al. [100] | Clinical assessment, CBCT scan | 3.5 | 8.7 | 5.2 | 4.2 | 100 | ||
| Beitlitum et al. [117] | Clinical assessment, CBCT scan | 5.8 ± 0.6 | 10.0 ± 1.4 | 4.2 ± 0.9 | – | 0 | 100/24 mo | |
| Di Stefano et al. [104] | Clinical assessment, CT scan, OPG, histology, immunohistochemistry | 3.9 ± 0.1 | 7.1 ± 0.2 | 3.2 | 35 | 0 | 100 | |
| Nissan et al. [103] | Clinical assessment; CBCT scan; OPG | – | – | 5.6 ± 1.0 | 0.2 ± 0.2 | – | 20.7 | 95.3/37 mo |
| Schwartz-Arad et al. [99] | Clinical assessment; OPG, CT scan | – | – | – | – | – | 3.6 |
98.5/12 mo 92.5/36 mo 77.5/48 mo |
| Silva et al. [105] | Clinical, histology, microtomographic morphometry | – | – | 4.6 ± 1.3 | 0.6 | 31.8 | 0 | 96/31.8 mo |
| Urban et al. [102] | Clinical assessment; periapical radiographs histomorphometry in 9 sites | 1.9 | 7.2 | 5.3 | 1.1 | 31 | 6.3 | 100 |
Complications %: number of patients complication rate in the augmented sites occurring during the observation period; implant survival %: survival rate of implants in the augmented area in percent; horizontal gain (mm): horizontal augmentation result at the end of the observation period in millimeters; horizontal width (mm): horizontal metrics at the end of the observation period in millimeters; loss (mm)/(%): difference between the initially augmented distance and the final result in millimeters/percent; bone formation (%): amount of newly formed bone in the defect area in percent
The majority of the included patients were female. Where specified, individuals were 70 women (72.2%) compared to 27 men (27.8%). The inclusion criteria for augmentation procedures and subsequent implant therapy did also vary, as for instance in some studies smokers were explicitly excluded from the study and in other studies smoking status of the patients was only recorded (Table 5). In all studies, the systemic health status was addressed in the inclusion criteria. However, the parameters ranged from patients being “in good health” to listing of specific systemic medical conditions such as “connective tissue diseases” and “immunodeficiencies”.
Table 5.
Patient characteristics and implant specification
| Author | Gender | Age (y) | Inclusion criteria | Exclusion criteria | Smoking | Systemic condition | Implant placement after augmentation (mths) | Types of implants | Mean follow-up (mths) |
|---|---|---|---|---|---|---|---|---|---|
| Amorfini et al. [101] | – | Median 59.5 (32–72) | Adult patients; bilateral atrophic edentulous areas in the posterior mandible | Systemic diseases affecting the bone metabolism | n/a | No systemic condition affecting bone metabolism | Simultaneous | SLActive; Straumann (Basel, Switzerland) | 12 |
| Barbu et al. [100] | 13 female; 11 male | Mean 47.8 (24–71) | At least 10-mm residual height, but less than 4.3-mm bucco-lingual dimension | Smoking; uncontrolled systemic disease; active periodontal disease | Excluded | No uncontrolled systemic disease | 4 | TSV Zimmer Dental Inc (Carlsbad, California, USA) | 19.8 |
| Beitlitum et al. [117] | 12 female; 3 male | Mean 53 (36–68) | Mandibular partial edentulism, horizontal bone deficiency that prevented implant placement | Presence of uncontrolled periodontal disease involving the residual dentition, an active endodontic condition involving the adjacent teeth | > 10cig/d excluded | No pregnancy or lactation, bone disease or medication interfering with bone metabolism, history of head and neck radiotherapy, metabolic disorders (eg, uncontrolled diabetes mellitus), immunodeficiency | 5–7 | – | 6 |
| Di Stefano et al. [104] | 3 female; 2 male | Mean 45.5 (32–59) | Crestal width ≤ 4 mm, crestal height ≥ 10 mm, age > 30 years, controlled oral hygiene and absence of lesions | High degree of bruxism; excessive consumption of alcohol; localized radiation therapy of the oral cavity; inflammatory and autoimmune diseases of the oral cavity; and poor oral hygiene | > 10cig/d excluded | No antineoplastic chemotherapy; blood, liver, and kidney diseases; immunosuppression; corticosteroids and bisphosphonates therapy; pregnancy; | 6 | XiVE, Dentsply-Friadent, (Mannheim, Germany) | 40.5 |
| Nissan et al. [103] | 18 female; 3 male | Mean 55.7 | Patients with a mandibular alveolar ridge requiring a vertical and lateral augmentation of > 3 mm patients in good health | Contraindications to implant therapy | n/a | Healthy | 6 | Seven; MIS® (Savion, Israel) or Osseotite; Zimmer Biomet 3i (Warsaw, USA) | 37 ± 17 |
| Schwartz-Arad et al. [99] | – | 50.3 | Edentulous mandibular area with degree of atrophy preventing placement of implants of at least 6 mm in height without the risk of damaging anatomical structures | Poor oral hygiene noncompliance | 18.2% | Exclusion of kidney/liver/connective tissue disease, immunodeficiency, chemotherapy, radiotherapy | 5 | Screw-Vent and Spline; Zimmer Biomet 3i, (Warsaw, USA) or NobelActive and Replace Select, Nobel Biocare (Goteborg, Sweden) or Implant Direct (Zurich, Switzerland) | 39.9 ± 30.9 |
| Silva et al. [105] | 15 female; 5 male | Mean 51.8 (37–64) | Alveolar ridge width and/or height ≤ 6 mm; > 18 yrs. Good oral health, no active periodontal disease, or occlusal problems | Compromised oral or general health, pregnancy, alcohol abuse, radiotherapy, bisphosphonates | Excluded | Good general and mental health | 6 | TitamaxCM Cortical, Neodent, (Curitiba, Brazil) | 31.8 ± 7 |
| Urban et al. [102] | 9 female; 3 male | 51.4 | Horizontal ridge of 4 mm or less, Cawood Howell Class IV, ability to maintain good oral hygiene | Smokers, patients with alcohol abuse, periodontal disease | Excluded | Systemically and periodontally healthy | 6.9 | Implants with an anodized TiUnite surface (Brånemark System, Nobel Biocare) | 20.9 ± 9.5 |
The horizontal mandibular bone serving as indication for augmentation procedures in the studies was also reported in various ways. The reported projected bone increase needed for implant placement was 3 mm of bone width [103]. Other groups performed lateral augmentation in case of residual width from < 4 mm [102, 104] to < 6 mm [105]. The article of Schwarz-Arad et al. proposed a need for augmentation if an implant of 6 mm in height could not be placed without the risk of damaging anatomical structures. The horizontal gain of bone width after the healing process was evaluated radiographically in seven studies. A gain of horizontal bone width was reported in seven of eight studies, with a mean of 4.8 mm, ranging from 3.2 to 5.7 mm (Table 4).
No significant difference was found regarding the outcome variables due to the heterogeneity of reporting the data. The use of rhPDGF-BB in one study significantly limited the resorption of augmented bone [101]. This observation was true for both the group having been augmented with corticocancellous allograft of the iliac bone and the control group of bone chips from the retromolar region. Marginal bone loss during the healing period was evaluated in four studies, with a mean of 0.5 mm, and values between 0.2 and 1.1 mm. Histologic findings were reported in three studies [102, 104, 105]. In the study conducted by Urban et al., a xenograft was combined with autologous bone and the mandible was augmented using particulated material. Histological results showed autologous bone in 31.0%, ABBM, remaining xenograft in 25.8% and marrow space in 43.2% of the derived samples. Di Stefano et al. and Silva et al. quantified newly formed bone at 35.0% and 31.8%, respectively. None of the studies reported on implant success rates. Implant survival rates were shown to be above 92.5% in the included studies at 36 months of last follow-up (Table 4). Four studies reported on crestal incision techniques with an implant survival rate ranging from 92.5 to 100.0% at 18.9 to 37 months of follow-up. One study group used a lateral incision for the augmentation and the surgical approach was not specified in the remainder of the reports. The declared implant survival rate was 96% to 100% with a mean follow-up of 10 to 40.5 months.
One study reported up to 3.6% complications concerning the grafted site at the follow-up [101]. Although data of horizontal and combined horizontal–vertical expansion of the atrophic mandible have not been reported separately in the investigated work by Schwartz-Arad et al., the total complication rate of mandibular augmentation was found to be 6.1%. However, only two of the seven complications occurred in solely horizontal augmented sites. Failure of one graft [6.3%] was reported in the study by Urban et al., when looking at the data of mandibular augmented sites. A comparatively high failure rate of six bone grafts was observed in the study by Nissan et al. [20.7%]. Although distinct failure rates in the horizontally augmented mandible are missing, most of the allograft failures [71.0%] in this study occurred in the posterior mandible. Barbu and colleagues reported graft exposure 2 weeks after the augmentation in one patient. Another week later clinical signs of necrosis led to removal of the graft resulting in a failure rate of 4.2% [100]. In conjunction with bone graft failure rates of 0.0% in four reports, the overall average amounts to 4.4% across all studies.
Discussion
Successful long-time survival and success of dental implants depend on sufficient amount and quality of bone. In case of severe horizontal bone loss, horizontal ridge augmentation of the mandibular ridge can provide optimum conditions for successful implant placement. However, the jaw recipient site (maxilla or mandible) has been shown to influence graft resorption [106]. Therefore, the aim of the present review was to systematically examine the clinical efficacy of augmentation procedures in horizontally resorbed mandibular ridges in terms of horizontal bone gain, implant success and survival after a follow-up period of at least 6 months. The results of this systematic review indicate a high variability in types of interventions to gain horizontal bone width. However, all techniques were able to create a sufficient horizontal bone gain. Implant survival was very good with results exceeding 92.5% between 12 and 36 months of follow-up.
Even though the present review aims to report the results in a standardized way, the wide range of different settings, study protocols and outcome parameters prevent drawing conclusions in an evidence-based manner. Only eight studies met the inclusion criteria and could be considered for this review, which is one of the main limitations of this study. However, the authors chose not to further modify the inclusion criteria to provide sufficient evidence. Additional inherent limitations are different study designs, different materials used, different assessment methods and prominently different outcome measures throughout. Therefore, comparisons from study to study are limited and comprehensive statistical analysis was not feasible. Augmentation procedures always included the use of membranes. In all studies, combinations of materials (autologous bone combined with xenografts or allografts) were used. The minimum bone width to include patients was either not specified or differed from study to study. Only three of the evaluated studies were randomized [100, 101, 103]. None of the studies reported on implant success rates. This results in the demand of further studies particularly focusing on how implants survive. This also includes the claim on reporting prosthetic data that were proposed at the 4th EAO Consensus Conference [107].
As case reports or case series with less than five patients did not meet the criteria for inclusion in this review, a considerable part of the pre-selected publications (19 in total) had to be dismissed in the process of data extraction. An observation period falling below 6 months after augmentation was reported in eight studies and considered too short to evaluate augmentation procedure and implant success. A major shortcoming during thorough data extraction was whether the horizontal ridge augmentation took place in the upper or lower jaw. As it has been reported that the augmentation procedure in the mandible may be correlated with higher complication rates and thus might be less predictable, the need for reporting the data in this distinctive way remains high [108].
Overall results show that all bone grafts that were used in the included studies have the potential to increase horizontal bone width. Even though no analysis could be carried out due to the lack of homogeneous data and outcome results, the descriptive data suggest a slightly better performance of bone blocks for horizontal augmentation of the lower alveolar ridge than for particulate material. In contrast, Urban and colleagues found comparable results on bone block augmentations using particulate autologous bone grafts in combination with allogeneic material (horizontal bone gain of 5.3 mm in mean).
Troeltzsch et al. conducted a comprehensive pooled analysis with regard to clinical efficacy of grafting materials in alveolar ridge augmentation over a weighted mean follow-up of 27.4 months (range 3–168 months) [20]. In this review, the mean follow-up was 26.0 months across all included studies, with a maximum mean observation period of 40.5 months, and minimum mean observation period of 6 months. After augmentation, the weighted mean horizontal gain for all particulate grafting materials was 3.7 ± 1.2 mm, with variation between 2.2 ± 1.2 mm (synthetic) and 4.5 ± 1.0 mm (mixtures of autogenous bone with allogeneic/xenogeneic grafting material) without statistical significance in the work of Troeltzsch et al. The authors derive a horizontal gain of 4.5 ± 1.2 mm for block grafts. Limited to the inclusion criteria of our review, a gain of horizontal bone width of the mandible in seven of eight studies, with a mean of 4.8 mm, ranging from 3.2 to 5.7 mm was observed. All but one study, reported low bone graft failure rates of 4.4% in average. One group of authors, that used bone-block allografts combined with particulate mineralized freeze-dried bone allograft or particulate bovine bone xenografts, reported a failure rate of 20.7% [103]. The reasons were only discussed marginally, and the authors suggested a relation to the localization of the augmentation as all failures had occurred in the posterior mandible. However, a successful use of allografts was stated in further studies that did not meet the inclusion criteria of this review. This suggests that other reasons than the use of allografts might be responsible for the high failure rate in the above-mentioned study [105, 109].
The augmentation procedure and subsequent complication rates may also be associated with the surgical approach. Horizontal ridge augmentation techniques in the mandible have been shown to be very efficient and safe. However, the method of placing narrow-diameter implants in horizontally resorbed alveolar ridges provides an alternative approach when bone loss is limited. Studies showed that results of narrow-diameter implants placed to support single crowns in the posterior region of the jaw did not differ from results of regular implants regarding the outcome parameters of marginal bone level, implant survival and success rates [110]. Moreover, recent studies suggest that narrow-diameter implants (2.75 to 3.25 mm) can successfully be used as minimally invasive alternative to horizontal bone augmentation in the posterior mandible with implant survival rates exceeding 97% [111]. A recent clinical trial over 2 years showed that patients receiving mini implants with shorter diameters of 1.8–2.4 mm had clinical outcomes similar to those of patients receiving conventional dental implants to support overdenture prostheses [112]. These results must be taken into account when considering horizontal ridge augmentation procedures in the mandible. To appropriately evaluate lateral augmentation techniques in the mandible, other approaches like the split bone block technique had to be excluded in the present review. Given the lack of information provided in the studies, no recommendation can be given concerning the surgical approach.
All studies included the use of a barrier membrane. Due to missing control groups without membranes, a beneficial effect of this procedure on horizontal ridge preservation techniques accompanying augmentation is conceivable but cannot be evaluated. However, literature regarding the reconstruction of peri-implant dehiscence defects suggests a beneficial effect of barrier membranes on the degree of defect filling [20]. Long-term follow-up data based on imaging results are not consistently provided. According to the guidelines of Albrektsson et al., the majority of crestal bone remodeling occurs during the first 2 years following implant-loading, therefore long-term (at least 2 years) follow-up of implants inserted in the augmented sites would be advisable [3]. Furthermore, bleeding and deep peri-implant pockets with crestal bone loss are closely related to the faith of newly formed hard tissues, which are reconstructed via GBR. Thus, implant success rate, which depends on change of crestal bone level and the lack of inflammatory signs, may represent a more relevant evaluation method of GBR efficacy than implant survival rate during long-term follow-ups.
Considering the limited number of included studies and the various approaches that were used, it is not possible to give concise recommendations for horizontal ridge augmentation procedures. Data of prospective randomized and controlled clinical trials regarding different horizontal ridge augmentation techniques including longer follow-up intervals, standardized outcome measures and distinguished results for the upper and lower jaw are missing. As implant success was not evaluated in any of the present studies, further investigations must be conducted including data on how implants and prosthetic reconstructions survive over time.
The role of growth factors in horizontal ridge augmentation must be outlined in a more specific manner. Two of the included studies did in fact work with growth factors—rhPDGF and PRP/PPP, respectively. As the present review is not designed to outline the role of growth factors, no evidence-based conclusion can be drawn from the present data. However, it must be kept in mind that these growth factors present a bias in the present study, which must be considered when interpreting the results.
It is obvious that different surgeons prefer different surgical techniques according to their individual expertise. Therefore, a comparison of distinct GBR procedures in studies with a single surgeon in a split-mouth design hardly represents comparable data. Thus, randomized controlled trials might represent an objective approach with clinically relevant results if the procedures are performed by two different surgeons practicing their favorable techniques after randomization. This would filter the advantages against the individual skillset of the surgeon.
The primary objective can be concluded based on quantitative data, but from the secondary objectives only implant survival rate can be answered. It is of high interest whether the biological behavior of augmented sites is similar to pristine bone or not. Furthermore, bone block alone or bone block supported GBR techniques are compared with GBR techniques based on particulate bone and xenograft (composite graft). Due to the difficulties of these procedures, but also due to different extent and morphology of bone defects, it is very likely that the superiority of one therapy cannot be easily concluded. However, a standardized follow-up protocol with particular timing and assessment methods is needed for getting clear results regarding techniques and long-term implant success.
Data extraction and assessment were performed by two authors to reduce reviewer bias. However, this kind of bias cannot be ruled out completely. As there is a possibility that only studies were published with favorable outcomes or significant findings, a publication bias might exist. Accordingly, studies with a follow-up of less than 6 months could have been so-called “cancelled studies” due to a poor outcome and therefore are not recorded by the mentioned search strategy [80, 81]. As the data extraction is based solely on reported outcomes in the present studies, a reporting bias and incomplete outcome data might have been influenced the data assessment. Predominantly, in five of eight studies multiple biases exist regarding randomization and blinding within each study. Only one study by Amorfini et al. is reported to be a randomized clinical trial on the research topic, whereas the study by Nissan et al. uses randomization only for parameters like deficiency filling and the use of membranes [101, 103]. Barbu and colleagues divided patients between the use of particulate graft composition and membrane and the data were de-identified before research analysis [100]. No further specifics on blinding and randomization can be found. Possible risks of bias according to the Cochrane Collaboration’s tool for randomized controlled trials, as well as the ROBINS-I tool for prospective cohorts, and the Checklist for Case Series from the Joanna Briggs Institute are given in Table 2.
Materials and methods
Focused question and PICO
Does the outcome of horizontal mandibular augmentation using xenogeneic, synthetic, or allogeneic material differ from the outcome of autologous bone grafts with regard to gained quantity of bone width, implant survival, success and complication rate in patients that underwent resorption of the horizontal alveolar ridge? The PICO Question is depicted in Table 6.
Table 6.
PICO Question
| Population | Healthy patients that suffered from resorption of the horizontal alveolar ridge after tooth removal with the lack of possibility to place dental implants without alveolar ridge augmentation prior to or accompanying implant placement |
| Intervention | Horizontal ridge augmentation using autologous, xenogeneic, synthetic, or allogeneic material or combinations of these |
| Comparison | Horizontal augmentation using only autologous bone grafts |
| Outcome variables |
Primary outcome: gain of bone width Secondary outcomes: implant survival, success and complication rate |
Search strategy
A computerized literature search (Medline/PubMed and Cochrane Central Register of Controlled Trials (CENTRAL)) was performed for clinical studies, including articles published from January 1st, 1995, up to March 31st, 2021. Additionally, manual search was carried out in: International Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Craniomaxillofacial Surgery, Journal of Clinical Periodontology, Journal of Periodontology, Clinical Oral Implants Research, International Journal of Oral Maxillofacial Implants, Clinical Implant Dentistry and Related Research, Journal of Implantology, Journal of Biomedical Materials Research. Furthermore, full-text articles of reviews published between January 2012 and March 2021 were obtained. An additional manual search was performed on these reviews to identify relevant studies. The language was limited to English. Risk of bias for RCTs was assessed in agreement with the Cochrane Collaboration’s tool [113, 114]. For non-randomized studies, the ROBINS-I tool was applied, and the Checklist for Case Series from the Joanna Briggs Institute [115]. The study followed the PRISMA guidelines (see Additional file 1: Table S1) [116] and was prospectively registered in the international prospective register of systematic reviews (PROSPERO) under registration number CRD42018082149 (www.crd.york.ac.uk/PROSPERO/). No contact was made to acquire additional data from the authors of the study. Any data report and subsequent conclusions are made based solely on published reports.
Search terms
The following search terms were applied: (“xenogeneic bone substitute” OR “synthetic bone substitute” OR “allogeneic bone graft” OR “xenograft” OR “allograft” OR “synthetic material” OR “lateral space” OR “bone graft” OR “guided bone regeneration” OR “alveolar ridge augmentation” OR “dental augmentation” OR “ridge atrophy” OR “GBR” OR “horizontal augmentation success” OR “horizontal ridge augmentation” OR “bone substitute” OR “alveolar bone graft” OR “alveolar bone loss” OR “alveolar resorption”). The search was limited to “human trial”. Accordingly, the MESH Terms “clinical study”, “clinical trial”, “controlled clinical trial”, “randomized controlled trial” were used.
AND
(“dental implants” OR “lower jaw” OR “alveolar ridge” OR “implant site” OR “mandible”). The search was limited to the MESH Terms “Humans” and “Clinical Trial”.
Inclusion criteria
Publications were considered when all of the following criteria were applicable:
Human, clinical trials with a minimum of five patients reported upon.
Horizontal augmentation with bone grafts or substitute materials prior to or accompanying implant placement.
Follow-up of at least 6 months in mean.
Metric outcome measures following surgical intervention.
Exclusion criteria
Publications limited to (1–4) or containing (5–13) the following were excluded:
In vitro studies
Animal (preclinical) studies
Cadaver studies
Case reports and reports based on interviews and charts
Vascularized free bone grafts
Only soft tissue augmentation
Distraction osteogenesis
Socket preservation techniques
Augmentation procedures after removal of malignant or benign tumors
Patients receiving radio- or chemotherapy
Results of patients that had been presented in prior studies of the authors
Only vertical augmentation procedures
Other bone preservation procedures, e.g., splitting or spreading.
Selection of studies
Two researchers (CK and LM) independently screened the publications derived from the online search based on the inclusion criteria. Subsequently, the abstracts of the selected titles were obtained and screened for meeting the inclusion criteria. If an abstract was not available in the database, the abstract of the printed article was used. Full-text articles of the selected abstracts were obtained. Again, a selection was made based on the inclusion criteria for the full-text articles. For this purpose, “Materials and methods” and “Results” sections of these studies were investigated. No additional data were obtained other than data included in the articles.
Data extraction and method of analysis
Two researchers independently analyzed all data using data extraction tables (CK and LM). Information on the following parameters was extracted: author(s), year of publication, study setting and study design, patient cohort (age range, mean age, gender, drop outs), follow-up (mean time and range), type of grafting material (autologous, allogeneic, xenogeneic, synthetic), type of fixation, type of graft preparation (particulate vs. block graft), type of barrier material used (collagen based, PTFE, polymer, titanium, none), the regenerative objective (two-staged vs. simultaneous), test/control groups, information on smoking or systemic conditions of the patients as well as the timing of implant placement, the assessment method and complications during or after surgery.
Outcome parameters
Metric data and clinical outcome on the following parameters were extracted, as indicated:
horizontal width (mm)
horizontal gain (mm)
loss of augmented bone width (%)
complication rate of surgical and post-surgical complications (%)
bone formation (%)
implant survival rate (%).
The data extraction was defined as follows:
Complications (%): complication rate in the augmented sites occurring during the observation period in percent.
Implant survival (%): survival rate of implants in the augmented area in percent.
Horizontal width I (mm): horizontal bone width before augmentation in mm.
Horizontal width II (mm): entire horizontal bone width at the end of the observation period in mm.
Horizontal gain (mm): horizontal result of gained bone in mm at the end of the observation period.
Loss (mm)/(%): difference between the initially augmented bone width and the entire horizontal bone width in millimeters/percent.
Bone formation (%): the bone formation rate is the mineral apposition rate multiplied with the surface area undergoing bone formation. Amount of newly formed bone in the defect area was calculated in percent.
Conclusions
A variety of approaches and materials exist for the reconstruction of bone width of the mandible. Only limited evidence with risk of bias is available on the impact of using particular grafts or bone blocks for horizontal augmentation in the lower jaw. The results of this review show outcomes for xenogeneic as well as autologous bone materials for horizontal ridge augmentation of the lower jaw. The use of allogeneic bone-block grafts in combination with resorbable barrier membranes must be re-evaluated. Data of randomized controlled clinical trials indicating superiority of specific horizontal ridge augmentation procedures in the lower jaw are still missing and worthwhile being investigated.
Supplementary Information
Additional file 1: Table S1. PRISMA 2020 checklist.
Acknowledgements
Not applicable.
Abbreviations
- ABBM
Anorganic bovine bone-derived mineral
- BOP
Bleeding on probing
- BV
Bone volume changes
- CAL
Peri-implant clinical attachment level
- CCT
Controlled clinical trial
- CENTRAL
Cochrane Central Register of Controlled Trials
- ePTFE
Expanded polytetrafluoroethylene
- EX
Exposure
- EXH
Exposed site height
- EXW
Exposed site width
- GBR
Guided bone regeneration
- HA
Histological assessment
- IS
Implant survival
- mBL
Mean marginal bone loss
- ME
Membrane exposure
- PGF
Partial graft failure
- PI
Peri-implant plaque index
- PICO
Population, Intervention, Comparison, Outcome variable
- PPD
Peri-implant probing depth
- PRF
Platelet-rich fibrin
- RA
Radiographic assessment
- POP
Penetration of the probe
- PRP
Platelet-rich plasma
- RCT
Randomized controlled trial
- rh-BMP2
Recombinant human bone morphogenic protein 2
- rhPDGF-BB
Recombinant human platelet-derived growth factor-BB
- SP
Success rate procedure
- TGF
Total graft failure
- WG
Width gain
- WR
Width reduction
Author contributions
LM and CK screened, analyzed, and interpreted the data and wrote the original manuscript draft. AH and RS provided conceptualization and validation of the data. MG contributed resources and project administration. PW, RJ, NB, MS, JK and MP reviewed and edited the manuscript substantially. BAN provided project supervision and correspondence with the journal. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Smeets reports grants and lecture fees from CAMLOG Biotechnologies AG, lecture fees from Straumann, Dentsply and Botiss, outside the submitted work. Dr. Jung reports grants from CAMLOG Biotechnologies AG, during the conduct of the study and receiving grants, lecture fees from the following industrial companies: Geistlich, Straumann, Vita, Dentsply, Henry Schein. Dr. Al-Nawas reports grants and personal fees from Geistlich, Straumann, and Dentsply, grants from Nobel Biocare, outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ralf Smeets and Levi Matthies contributed equally to this work
References
- 1.Buser D, Sennerby L, De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017;73(1):7–21. doi: 10.1111/prd.12185. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan M, Meyer S, Mombelli A, Muller F. Dental implants in the elderly population: a systematic review and meta-analysis. Clin Oral Implants Res. 2017;28(8):920–930. doi: 10.1111/clr.12898. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Chrcanovic B, Ostman PO, Sennerby L. Initial and long-term crestal bone responses to modern dental implants. Periodontol 2000. 2017;73(1):41–50. doi: 10.1111/prd.12176. [DOI] [PubMed] [Google Scholar]
- 4.Chrcanovic BR, Albrektsson T, Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont. 2017;30(3):219–237. doi: 10.11607/ijp.5142. [DOI] [PubMed] [Google Scholar]
- 5.Smeets R, Henningsen A, Jung O, Heiland M, Hammacher C, Stein JM. Definition, etiology, prevention and treatment of peri-implantitis—a review. Head Face Med. 2014;10:34. doi: 10.1186/1746-160X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 7.Vignoletti F, Discepoli N, Muller A, de Sanctis M, Munoz F, Sanz M. Bone modelling at fresh extraction sockets: immediate implant placement versus spontaneous healing: an experimental study in the beagle dog. J Clin Periodontol. 2012;39(1):91–97. doi: 10.1111/j.1600-051X.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 8.Lutz R, Neukam FW, Simion M, Schmitt CM. Long-term outcomes of bone augmentation on soft and hard-tissue stability: a systematic review. Clin Oral Implants Res. 2015;26(Suppl 11):103–122. doi: 10.1111/clr.12635. [DOI] [PubMed] [Google Scholar]
- 9.Sanz-Sanchez I, Ortiz-Vigon A, Sanz-Martin I, Figuero E, Sanz M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: a systematic review and meta-analysis. J Dent Res. 2015;94(9 Suppl):128S–S142. doi: 10.1177/0022034515594780. [DOI] [PubMed] [Google Scholar]
- 10.Aghaloo TL, Misch C, Lin GH, Iacono VJ, Wang HL. Bone augmentation of the edentulous maxilla for implant placement: a systematic review. Int J Oral Maxillofac Implants. 2016;31(Suppl):s19–30. doi: 10.11607/jomi.16suppl.g1. [DOI] [PubMed] [Google Scholar]
- 11.Elnayef B, Monje A, Gargallo-Albiol J, Galindo-Moreno P, Wang HL, Hernandez-Alfaro F. Vertical ridge augmentation in the atrophic mandible: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2017;32(2):291–312. doi: 10.11607/jomi.4861. [DOI] [PubMed] [Google Scholar]
- 12.Bassetti MA, Bassetti RG, Bosshardt DD. The alveolar ridge splitting/expansion technique: a systematic review. Clin Oral Implants Res. 2016;27(3):310–324. doi: 10.1111/clr.12537. [DOI] [PubMed] [Google Scholar]
- 13.Klein MO, Schiegnitz E, Al-Nawas B. Systematic review on success of narrow-diameter dental implants. Int J Oral Maxillofac Implants. 2014;29(Suppl):43–54. doi: 10.11607/jomi.2014suppl.g1.3. [DOI] [PubMed] [Google Scholar]
- 14.Windisch P, Orban K, Salvi GE, Sculean A, Molnar B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: a prospective case series. Clin Oral Investig. 2020;25(5):2969–2980. doi: 10.1007/s00784-020-03617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemipoor M, Asghari N, Mohammadi M, Kalantari M, Arabsolghar M, Ranjbar H. Radiological and histological evaluation of horizontal ridge augmentation using corticocancellous freeze-dried bone allograft with and without autogenous bone: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2020;22(5):582–592. doi: 10.1111/cid.12935. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza-Azpur G, de la Fuente A, Chavez E, Valdivia E, Khouly I. Horizontal ridge augmentation with guided bone regeneration using particulate xenogenic bone substitutes with or without autogenous block grafts: a randomized controlled trial. Clin Implant Dent Relat Res. 2019;21(4):521–530. doi: 10.1111/cid.12740. [DOI] [PubMed] [Google Scholar]
- 17.Meloni SM, Jovanovic SA, Urban I, Baldoni E, Pisano M, Tallarico M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1: 1 ratio of particulate xenograft and autologous bone: a 3-year after final loading prospective clinical study. Clin Implant Dent Relat Res. 2019;21(4):669–677. doi: 10.1111/cid.12808. [DOI] [PubMed] [Google Scholar]
- 18.Spin-Neto R, Stavropoulos A, Dias Pereira LA, Marcantonio E, Jr, Wenzel A. Fate of autologous and fresh-frozen allogeneic block bone grafts used for ridge augmentation. A CBCT-based analysis. Clin Oral Implants Res. 2013;24(2):167–173. doi: 10.1111/j.1600-0501.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- 19.Maiorana C, Beretta M, Salina S, Santoro F. Reduction of autogenous bone graft resorption by means of bio-oss coverage: a prospective study. Int J Periodontics Restor Dent. 2005;25(1):19–25. [PubMed] [Google Scholar]
- 20.Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, et al. Clinical efficacy of grafting materials in alveolar ridge augmentation: a systematic review. J Craniomaxillofac Surg. 2016;44(10):1618–1629. doi: 10.1016/j.jcms.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Bell RB, Blakey GH, White RP, Hillebrand DG, Molina A. Staged reconstruction of the severely atrophic mandible with autogenous bone graft and endosteal implants. J Oral Maxillofac Surg. 2002;60(10):1135–1141. doi: 10.1053/joms.2002.34986. [DOI] [PubMed] [Google Scholar]
- 22.Cotter CJ, Maher A, Gallagher C, Sleeman D. Mandibular lower border: donor site of choice for alveolar grafting. Br J Oral Maxillofac Surg. 2002;40(5):429–432. doi: 10.1016/S0266-4356(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 23.Ella B, Laurentjoye M, Sedarat C, Coutant JC, Masson E, Rouas A. Mandibular ridge expansion using a horizontal bone-splitting technique and synthetic bone substitute: an alternative to bone block grafting? Int J Oral Maxillofac Implants. 2014;29(1):135–140. doi: 10.11607/jomi.2201. [DOI] [PubMed] [Google Scholar]
- 24.Elo JA, Herford AS, Boyne PJ. Implant success in distracted bone versus autogenous bone-grafted sites. J Oral Implantol. 2009;35(4):181–184. doi: 10.1563/1548-1336-35.4.181. [DOI] [PubMed] [Google Scholar]
- 25.Hu GH, Froum SJ, Alodadi A, Nose F, Yu YP, Suzuki T, et al. A three-stage split-crest technique: case series of horizontal ridge augmentation in the atrophic posterior mandible. Int J Periodontics Restor Dent. 2018;38(4):565–573. doi: 10.11607/prd.2907. [DOI] [PubMed] [Google Scholar]
- 26.Huang HY, Ogata Y, Hanley J, Finkelman M, Hur Y. Crestal bone resorption in augmented bone using mineralized freeze-dried bone allograft or pristine bone during submerged implant healing: a prospective study in humans. Clin Oral Implants Res. 2016;27(2):e25–e30. doi: 10.1111/clr.12512. [DOI] [PubMed] [Google Scholar]
- 27.Keith JD, Jr, Petrungaro P, Leonetti JA, Elwell CW, Zeren KJ, Caputo C, et al. Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period (2001–2004) Int J Periodontics Restor Dent. 2006;26(4):321–327. [PubMed] [Google Scholar]
- 28.Stellingsma K, Raghoebar GM, Meijer HJ, Stegenga B. The extremely resorbed mandible: a comparative prospective study of 2-year results with 3 treatment strategies. Int J Oral Maxillofac Implants. 2004;19(4):563–577. [PubMed] [Google Scholar]
- 29.Stellingsma K, Raghoebar GM, Visser A, Vissink A, Meijer HJ. The extremely resorbed mandible, 10-year results of a randomized controlled trial on 3 treatment strategies. Clin Oral Implants Res. 2014;25(8):926–932. doi: 10.1111/clr.12184. [DOI] [PubMed] [Google Scholar]
- 30.Sohn DS, Lee HJ, Heo JU, Moon JW, Park IS, Romanos GE. Immediate and delayed lateral ridge expansion technique in the atrophic posterior mandibular ridge. J Oral Maxillofac Surg. 2010;68(9):2283–2290. doi: 10.1016/j.joms.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Rachmiel A, Emodi O, Aizenbud D, Rachmiel D, Shilo D. Two-stage reconstruction of the severely deficient alveolar ridge: bone graft followed by alveolar distraction osteogenesis. Int J Oral Maxillofac Surg. 2018;47(1):117–124. doi: 10.1016/j.ijom.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Thalmair T, Fickl S, Schneider D, Hinze M, Wachtel H. Dimensional alterations of extraction sites after different alveolar ridge preservation techniques—a volumetric study. J Clin Periodontol. 2013;40(7):721–727. doi: 10.1111/jcpe.12111. [DOI] [PubMed] [Google Scholar]
- 33.Torres J, Tamimi F, Alkhraisat MH, Manchon A, Linares R, Prados-Frutos JC, et al. Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J Clin Periodontol. 2010;37(10):943–951. doi: 10.1111/j.1600-051X.2010.01615.x. [DOI] [PubMed] [Google Scholar]
- 34.Thanasrisuebwong P, Kiattavorncharoen S, Deeb GR, Bencharit S. Implant site preparation application of injectable platelet-rich fibrin for vertical and horizontal bone regeneration: a clinical report. J Oral Implantol. 2020;48(1):43–50. doi: 10.1563/aaid-joi-D-20-00031. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Chen L, Ning Y, Ding X, Gao Y, Zhou L, et al. Split-crest technique with inlay bone block grafts for narrow posterior mandibles: a retrospective clinical study with a 3-year follow-up. Am J Transl Res. 2020;12(8):4628–4638. [PMC free article] [PubMed] [Google Scholar]
- 36.Chaushu L, Chaushu G, Kolerman R, Vered M, Naishlos S, Nissan J. Anterior atrophic mandible restoration using cancellous bone block allograft. Clin Implant Dent Relat Res. 2019;21(5):903–909. doi: 10.1111/cid.12744. [DOI] [PubMed] [Google Scholar]
- 37.Bazrafshan N, Darby I. Retrospective success and survival rates of dental implants placed with simultaneous bone augmentation in partially edentulous patients. Clin Oral Implants Res. 2014;25(7):768–773. doi: 10.1111/clr.12185. [DOI] [PubMed] [Google Scholar]
- 38.Beitlitum I, Artzi Z, Nemcovsky CE. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin Oral Implants Res. 2010;21(11):1242–1250. doi: 10.1111/j.1600-0501.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- 39.Buser D, Ingimarsson S, Dula K, Lussi A, Hirt HP, Belser UC. Long-term stability of osseointegrated implants in augmented bone: a 5-year prospective study in partially edentulous patients. Int J Periodontics Restor Dent. 2002;22(2):109–117. [PubMed] [Google Scholar]
- 40.Chappuis V, Cavusoglu Y, Buser D, von Arx T. Lateral ridge augmentation using autogenous block grafts and guided bone regeneration: a 10-year prospective case series study. Clin Implant Dent Relat Res. 2017;19(1):85–96. doi: 10.1111/cid.12438. [DOI] [PubMed] [Google Scholar]
- 41.Dahlin C, Simion M, Hatano N. Long-term follow-up on soft and hard tissue levels following guided bone regeneration treatment in combination with a xenogeneic filling material: a 5-year prospective clinical study. Clin Implant Dent Relat Res. 2010;12(4):263–270. doi: 10.1111/j.1708-8208.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 42.de Lacerda PE, Pelegrine AA, Teixeira ML, Montalli VA, Rodrigues H, Napimoga MH. Homologous transplantation with fresh-frozen bone for dental implant placement can induce HLA sensitization: a preliminary study. Cell Tissue Bank. 2016;17(3):465–472. doi: 10.1007/s10561-016-9562-9. [DOI] [PubMed] [Google Scholar]
- 43.Hellem S, Astrand P, Stenstrom B, Engquist B, Bengtsson M, Dahlgren S. Implant treatment in combination with lateral augmentation of the alveolar process: a 3-year prospective study. Clin Implant Dent Relat Res. 2003;5(4):233–240. doi: 10.1111/j.1708-8208.2003.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 44.Jung RE, Benic GI, Scherrer D, Hammerle CH. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures—a randomized, controlled clinical trial. Clin Oral Implants Res. 2013;26(1):28–34. doi: 10.1111/clr.12296. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto I, Funaki K, Yamauchi K, Kodama T, Takahashi T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant Dent Relat Res. 2012;14(2):304–311. doi: 10.1111/j.1708-8208.2009.00257.x. [DOI] [PubMed] [Google Scholar]
- 46.Penarrocha-Diago M, Aloy-Prosper A, Penarrocha-Oltra D, Guirado JL, Penarrocha-Diago M. Localized lateral alveolar ridge augmentation with block bone grafts: simultaneous versus delayed implant placement: a clinical and radiographic retrospective study. Int J Oral Maxillofac Implants. 2013;28(3):846–853. doi: 10.11607/jomi.2964. [DOI] [PubMed] [Google Scholar]
- 47.Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237–247. doi: 10.1563/1548-1336(2006)32[237:UOTMFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Santana RB, Santana CM. A clinical comparison of guided bone regeneration with platelet-derived growth factor-enhanced bone ceramic versus autogenous bone block grafting. Int J Oral Maxillofac Implants. 2015;30(3):700–706. doi: 10.11607/jomi.3529. [DOI] [PubMed] [Google Scholar]
- 49.Sbordone L, Toti P, Menchini-Fabris G, Sbordone C, Guidetti F. Implant survival in maxillary and mandibular osseous onlay grafts and native bone: a 3-year clinical and computerized tomographic follow-up. Int J Oral Maxillofac Implants. 2009;24(4):695–703. [PubMed] [Google Scholar]
- 50.Spin-Neto R, Stavropoulos A, Coletti FL, Faeda RS, Pereira LA, Marcantonio E., Jr Graft incorporation and implant osseointegration following the use of autologous and fresh-frozen allogeneic block bone grafts for lateral ridge augmentation. Clin Oral Implants Res. 2014;25(2):226–233. doi: 10.1111/clr.12107. [DOI] [PubMed] [Google Scholar]
- 51.Wessing B, Emmerich M, Bozkurt A. Horizontal ridge augmentation with a novel resorbable collagen membrane: a retrospective analysis of 36 consecutive patients. Int J Periodontics Restor Dent. 2016;36(2):179–187. doi: 10.11607/prd.2065. [DOI] [PubMed] [Google Scholar]
- 52.Hattingh A, De Bruyn H, Van Weehaeghe M, Hommez G, Vandeweghe S. Contour changes following immediate placement of ultra-wide implants in molar extraction sockets without bone grafting. J Clin Med. 2020;9(8):2504. doi: 10.3390/jcm9082504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanco J, Alonso A, Sanz M. Long-term results and survival rate of implants treated with guided bone regeneration: a 5-year case series prospective study. Clin Oral Implants Res. 2005;16(3):294–301. doi: 10.1111/j.1600-0501.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 54.Buser D, Dula K, Lang NP, Nyman S. Long-term stability of osseointegrated implants in bone regenerated with the membrane technique. 5-year results of a prospective study with 12 implants. Clin Oral Implants Res. 1996;7(2):175–183. doi: 10.1034/j.1600-0501.1996.070212.x. [DOI] [PubMed] [Google Scholar]
- 55.Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res. 1999;10(4):278–288. doi: 10.1034/j.1600-0501.1999.100404.x. [DOI] [PubMed] [Google Scholar]
- 56.De Boever AL, De Boever JA. Guided bone regeneration around non-submerged implants in narrow alveolar ridges: a prospective long-term clinical study. Clin Oral Implants Res. 2005;16(5):549–556. doi: 10.1111/j.1600-0501.2005.01154.x. [DOI] [PubMed] [Google Scholar]
- 57.Eskan MA, Greenwell H, Hill M, Morton D, Vidal R, Shumway B, et al. Platelet-rich plasma-assisted guided bone regeneration for ridge augmentation: a randomized, controlled clinical trial. J Periodontol. 2014;85(5):661–668. doi: 10.1902/jop.2013.130260. [DOI] [PubMed] [Google Scholar]
- 58.Hammerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008;19(1):19–25. doi: 10.1111/j.1600-0501.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 59.Merli M, Moscatelli M, Mariotti G, Pagliaro U, Breschi L, Mazzoni A, et al. Membranes and bone substitutes in a one-stage procedure for horizontal bone augmentation: a histologic double-blind parallel randomized controlled trial. Int J Periodontics Restor Dent. 2015;35(4):463–471. doi: 10.11607/prd.2418. [DOI] [PubMed] [Google Scholar]
- 60.Mordenfeld A, Johansson CB, Albrektsson T, Hallman M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin Oral Implants Res. 2014;25(3):310–320. doi: 10.1111/clr.12143. [DOI] [PubMed] [Google Scholar]
- 61.Novell J, Novell-Costa F, Ivorra C, Farinas O, Munilla A, Martinez C. Five-year results of implants inserted into freeze-dried block allografts. Implant Dent. 2012;21(2):129–135. doi: 10.1097/ID.0b013e31824bf99f. [DOI] [PubMed] [Google Scholar]
- 62.Pendarvis WT, Sandifer JB. Localized ridge augmentation using a block allograft with subsequent implant placement: a case series. Int J Periodontics Restor Dent. 2008;28(5):509–515. [PubMed] [Google Scholar]
- 63.Proussaefs P, Lozada J. The use of resorbable collagen membrane in conjunction with autogenous bone graft and inorganic bovine mineral for buccal/labial alveolar ridge augmentation: a pilot study. J Prosthet Dent. 2003;90(6):530–538. doi: 10.1016/S0022-3913(03)00521-3. [DOI] [PubMed] [Google Scholar]
- 64.Proussaefs P, Lozada J, Kleinman A, Rohrer MD, McMillan PJ. The use of titanium mesh in conjunction with autogenous bone graft and inorganic bovine bone mineral (bio-oss) for localized alveolar ridge augmentation: a human study. Int J Periodontics Restor Dent. 2003;23(2):185–195. [PubMed] [Google Scholar]
- 65.Soehardi A, Meijer GJ, Strooband VF, de Koning M, Stoelinga PJ. The potential of the horizontal ramus of the mandible as a donor site for block and particular grafts in pre-implant surgery. Int J Oral Maxillofac Surg. 2009;38(11):1173–1178. doi: 10.1016/j.ijom.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Strietzel FP, Reichart PA, Graf HL. Lateral alveolar ridge augmentation using a synthetic nano-crystalline hydroxyapatite bone substitution material (Ostim): preliminary clinical and histological results. Clin Oral Implants Res. 2007;18(6):743–751. doi: 10.1111/j.1600-0501.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 67.von Arx T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10(1):24–33. doi: 10.1034/j.1600-0501.1999.100104.x. [DOI] [PubMed] [Google Scholar]
- 68.Zita Gomes R, Paraud Freixas A, Han CH, Bechara S, Tawil I. Alveolar ridge reconstruction with titanium meshes and simultaneous implant placement: a retrospective, multicenter clinical study. Biomed Res Int. 2016;2016:5126838. doi: 10.1155/2016/5126838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Landsberg C, Moses O. Ridge augmentation using customized allogeneic bone block: a 3-year follow-up of two case reports. Int J Periodontics Restor Dent. 2020;40(6):881–889. doi: 10.11607/prd.3354. [DOI] [PubMed] [Google Scholar]
- 70.Zhou M, Li SY, Terheyden H, Cao SS, Che YJ, Geng YM. Particulate coral hydroxyapatite sheltered by titanium mesh for localized alveolar rehabilitation after onlay graft failure: a case report. J Oral Implantol. 2018;44(2):147–152. doi: 10.1563/aaid-joi-D-17-00109. [DOI] [PubMed] [Google Scholar]
- 71.Mello BF, de Carvalho Formiga M, de Souza da Silva LF, Dos Santos Coura G, Shibli JA. Horizontal ridge augmentation using a xenograft bone substitute for implant-supported fixed rehabilitation: a case report with four years of follow-up. Case Rep Dent. 2020;2020:6723936. doi: 10.1155/2020/6723936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acocella A, Ercoli C, Geminiani A, Feng C, Billi M, Acocella G, et al. Clinical evaluation of immediate loading of electroeroded screw-retained titanium fixed prostheses supported by tilted implant: a multicenter retrospective study. Clin Implant Dent Relat Res. 2012;14(Suppl 1):e98–108. doi: 10.1111/j.1708-8208.2011.00379.x. [DOI] [PubMed] [Google Scholar]
- 73.D'Amato S, Tartaro G, Itro A, Nastri L, Santagata M. Block versus particulate/titanium mesh for ridge augmentation for mandibular lateral incisor defects: clinical and histologic analysis. Int J Periodontics Restor Dent. 2015;35(1):e1–8. doi: 10.11607/prd.2073. [DOI] [PubMed] [Google Scholar]
- 74.Friedmann A, Strietzel FP, Maretzki B, Pitaru S, Bernimoulin JP. Histological assessment of augmented jaw bone utilizing a new collagen barrier membrane compared to a standard barrier membrane to protect a granular bone substitute material. Clin Oral Implants Res. 2002;13(6):587–594. doi: 10.1034/j.1600-0501.2002.130603.x. [DOI] [PubMed] [Google Scholar]
- 75.Fu JH, Oh TJ, Benavides E, Rudek I, Wang HL. A randomized clinical trial evaluating the efficacy of the sandwich bone augmentation technique in increasing buccal bone thickness during implant placement surgery: I. Clinical and radiographic parameters. Clin Oral Implants Res. 2014;25(4):458–467. doi: 10.1111/clr.12171. [DOI] [PubMed] [Google Scholar]
- 76.Geurs NC, Korostoff JM, Vassilopoulos PJ, Kang TH, Jeffcoat M, Kellar R, et al. Clinical and histologic assessment of lateral alveolar ridge augmentation using a synthetic long-term bioabsorbable membrane and an allograft. J Periodontol. 2008;79(7):1133–1140. doi: 10.1902/jop.2008.070595. [DOI] [PubMed] [Google Scholar]
- 77.Peleg M, Sawatari Y, Marx RN, Santoro J, Cohen J, Bejarano P, et al. Use of corticocancellous allogeneic bone blocks for augmentation of alveolar bone defects. Int J Oral Maxillofac Implants. 2010;25(1):153–162. [PubMed] [Google Scholar]
- 78.Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54(4):420–432. doi: 10.1016/S0278-2391(96)90113-5. [DOI] [PubMed] [Google Scholar]
- 79.Atef M, Osman AH, Hakam M. Autogenous interpositional block graft vs onlay graft for horizontal ridge augmentation in the mandible. Clin Implant Dent Relat Res. 2019;21(4):678–685. doi: 10.1111/cid.12809. [DOI] [PubMed] [Google Scholar]
- 80.Ortiz-Vigon A, Suarez I, Martinez-Villa S, Sanz-Martin I, Bollain J, Sanz M. Safety and performance of a novel collagenated xenogeneic bone block for lateral alveolar crest augmentation for staged implant placement. Clin Oral Implants Res. 2018;29(1):36–45. doi: 10.1111/clr.13036. [DOI] [PubMed] [Google Scholar]
- 81.Angermair J, Bosshardt DD, Nelson K, Flügge TV, Stricker A, Fretwurst T. Horizontal bone grafting using equine-derived cancellous bone blocks is associated with severe complications: a prospective clinical and histological pilot study. Clin Oral Implants Res. 2020;31(11):1149–1158. doi: 10.1111/clr.13661. [DOI] [PubMed] [Google Scholar]
- 82.Chiapasco M, Colletti G, Romeo E, Zaniboni M, Brusati R. Long-term results of mandibular reconstruction with autogenous bone grafts and oral implants after tumor resection. Clin Oral Implants Res. 2008;19(10):1074–1080. doi: 10.1111/j.1600-0501.2008.01542.x. [DOI] [PubMed] [Google Scholar]
- 83.Khojasteh A, Hassani A, Motamedian SR, Saadat S, Alikhasi M. Cortical bone augmentation versus nerve lateralization for treatment of atrophic posterior mandible: a retrospective study and review of literature. Clin Implant Dent Relat Res. 2016;18(2):342–359. doi: 10.1111/cid.12317. [DOI] [PubMed] [Google Scholar]
- 84.Lenzen C, Meiss A, Bull HG. Augmentation of the extremely atrophied maxilla and mandible by autologous calvarial bone transplantation. Mund Kiefer Gesichtschir. 1999;3(Suppl 1):S40–S42. doi: 10.1007/PL00014514. [DOI] [PubMed] [Google Scholar]
- 85.Nissan J, Marilena V, Gross O, Mardinger O, Chaushu G. Histomorphometric analysis following augmentation of the posterior mandible using cancellous bone-block allograft. J Biomed Mater Res A. 2011;97(4):509–513. doi: 10.1002/jbm.a.33096. [DOI] [PubMed] [Google Scholar]
- 86.Ozkan Y, Ozcan M, Varol A, Akoglu B, Ucankale M, Basa S. Resonance frequency analysis assessment of implant stability in labial onlay grafted posterior mandibles: a pilot clinical study. Int J Oral Maxillofac Implants. 2007;22(2):235–242. [PubMed] [Google Scholar]
- 87.Sbordone L, Toti P, Menchini-Fabris GB, Sbordone C, Piombino P, Guidetti F. Volume changes of autogenous bone grafts after alveolar ridge augmentation of atrophic maxillae and mandibles. Int J Oral Maxillofac Surg. 2009;38(10):1059–1065. doi: 10.1016/j.ijom.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 88.Teng F, Zhang Q, Wu M, Rachana S, Ou G. Clinical use of ridge-splitting combined with ridge expansion osteotomy, sandwich bone augmentation, and simultaneous implantation. Br J Oral Maxillofac Surg. 2014;52(8):703–708. doi: 10.1016/j.bjoms.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 89.Toscano N, Holtzclaw D, Mazor Z, Rosen P, Horowitz R, Toffler M. Horizontal ridge augmentation utilizing a composite graft of demineralized freeze-dried allograft, mineralized cortical cancellous chips, and a biologically degradable thermoplastic carrier combined with a resorbable membrane: a retrospective evaluation of 73 consecutively treated cases from private practices. J Oral Implantol. 2010;36(6):467–474. doi: 10.1563/AAID-JOI-D-09-00100. [DOI] [PubMed] [Google Scholar]
- 90.Urban IA, Nagursky H, Lozada JL. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: a prospective case series in 22 patients. Int J Oral Maxillofac Implants. 2011;26(2):404–414. [PubMed] [Google Scholar]
- 91.Van Assche N, Michels S, Naert I, Quirynen M. Randomized controlled trial to compare two bone substitutes in the treatment of bony dehiscences. Clin Implant Dent Relat Res. 2013;15(4):558–568. doi: 10.1111/j.1708-8208.2011.00408.x. [DOI] [PubMed] [Google Scholar]
- 92.Viscioni A, Rigo L, Franco M, Brunelli G, Avantaggiato A, Sollazzo V, et al. Reconstruction of severely atrophic jaws using homografts and simultaneous implant placement: a retrospective study. J Oral Implantol. 2010;36(2):131–139. doi: 10.1563/AAID-JOI-D-09-00025. [DOI] [PubMed] [Google Scholar]
- 93.Korzh DG, Haritonov DY, Stepanov IV, Podoprigora AV. Evaluation of autogenous mandibular bone block resorption in horizontal alveolar ridge augmentation. Stomatologiia (Mosk) 2019;98(6):30–32. doi: 10.17116/stomat20199806130. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Su W, Xie X, Lei L, Bao J, He S, et al. A novel in situ bone elevation method to achieve vertical periodontal augmentation in dogs: a pilot study. J Oral Rehabil. 2019;46(8):756–764. doi: 10.1111/joor.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeon YT, Han SJ. Comparative study of the effect of paranasal augmentation with autologous bone in orthognathic surgery. J Oral Maxillofac Surg. 2019;77(10):2116–2124. doi: 10.1016/j.joms.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 96.Sanz-Martin I, Ferrantino L, Vignoletti F, Nuñez J, Baldini N, Duvina M, et al. Contour changes after guided bone regeneration of large non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a porcine-derived collagen membrane: an experimental in vivo investigation. Clin Oral Investig. 2018;22(3):1273–1283. doi: 10.1007/s00784-017-2214-z. [DOI] [PubMed] [Google Scholar]
- 97.Wang S, Wu W, Liu Y, Wang X, Tang L, You P, et al. Bone augmentation of peri-implant dehiscence defects using multilaminated small intestinal submucosa as a barrier membrane: an experimental study in dogs. Biomed Res Int. 2019;2019:8962730. doi: 10.1155/2019/8962730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JT, Cha JK, Kim S, Jung UW, Thoma DS, Jung RE. Lateral onlay grafting using different combinations of soft-type synthetic block grafts and resorbable collagen membranes: an experimental in vivo study. Clin Oral Implants Res. 2020;31(4):303–314. doi: 10.1111/clr.13566. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz-Arad D, Ofec R, Eliyahu G, Ruban A, Sterer N. Long term follow-up of dental implants placed in autologous onlay bone graft. Clin Implant Dent Relat Res. 2016;18(3):449–461. doi: 10.1111/cid.12288. [DOI] [PubMed] [Google Scholar]
- 100.Barbu HM, Andreescu CF, Lorean A, Kolerman R, Moraru L, Mortellaro C, et al. Comparison of two techniques for lateral ridge augmentation in mandible with ramus block graft. J Craniofac Surg. 2016;27(3):662–667. doi: 10.1097/SCS.0000000000002561. [DOI] [PubMed] [Google Scholar]
- 101.Amorfini L, Migliorati M, Signori A, Silvestrini-Biavati A, Benedicenti S. Block allograft technique versus standard guided bone regeneration: a randomized clinical trial. Clin Implant Dent Relat Res. 2014;16(5):655–667. doi: 10.1111/cid.12040. [DOI] [PubMed] [Google Scholar]
- 102.Urban IA, Nagursky H, Lozada JL, Nagy K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 25 patients. Int J Periodontics Restor Dent. 2013;33(3):299–307. doi: 10.11607/prd.1407. [DOI] [PubMed] [Google Scholar]
- 103.Nissan J, Ghelfan O, Mardinger O, Calderon S, Chaushu G. Efficacy of cancellous block allograft augmentation prior to implant placement in the posterior atrophic mandible. Clin Implant Dent Relat Res. 2011;13(4):279–285. doi: 10.1111/j.1708-8208.2009.00219.x. [DOI] [PubMed] [Google Scholar]
- 104.Di Stefano DA, Artese L, Iezzi G, Piattelli A, Pagnutti S, Piccirilli M, et al. Alveolar ridge regeneration with equine spongy bone: a clinical, histological, and immunohistochemical case series. Clin Implant Dent Relat Res. 2009;11(2):90–100. doi: 10.1111/j.1708-8208.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 105.Silva ER, Ferraz EP, Neto EC, Chaushu G, Chaushu L, Xavier SP. Volumetric stability of fresh frozen bone blocks in atrophic posterior mandible augmentation. J Oral Implantol. 2017;43(1):25–32. doi: 10.1563/aaid-joi-D-16-00095. [DOI] [PubMed] [Google Scholar]
- 106.Naenni N, Lim HC, Papageorgiou SN, Hämmerle CHF. Efficacy of lateral bone augmentation prior to implant placement: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(Suppl 21):287–306. doi: 10.1111/jcpe.13052. [DOI] [PubMed] [Google Scholar]
- 107.Klinge B, Flemming T, Cosyn J, De Bruyn H, Eisner BM, Hultin M, et al. The patient undergoing implant therapy. Summary and consensus statements. The 4th EAO consensus conference 2015. Clin Oral Implants Res. 2015;26(Suppl 11):64–67. doi: 10.1111/clr.12675. [DOI] [PubMed] [Google Scholar]
- 108.Bahat O, Fontanessi RV. Efficacy of implant placement after bone grafting for three-dimensional reconstruction of the posterior jaw. Int J Periodontics Restor Dent. 2001;21(3):220–231. [PubMed] [Google Scholar]
- 109.Ahmadi RS, Sayar F, Rakhshan V, Iranpour B, Jahanbani J, Toumaj A, et al. Clinical and histomorphometric assessment of lateral alveolar ridge augmentation using a corticocancellous freeze-dried allograft bone block. J Oral Implantol. 2017;43(3):202–210. doi: 10.1563/aaid-joi-D-16-00042. [DOI] [PubMed] [Google Scholar]
- 110.de Souza AB, Sukekava F, Tolentino L, Cesar-Neto JB, Garcez-Filho J, Araujo MG. Narrow- and regular-diameter implants in the posterior region of the jaws to support single crowns: a 3-year split-mouth randomized clinical trial. Clin Oral Implants Res. 2017;29(1):100–107. doi: 10.1111/clr.13076. [DOI] [PubMed] [Google Scholar]
- 111.Grandi T, Svezia L, Grandi G. Narrow implants (2.75 and 3.25 mm diameter) supporting a fixed splinted prostheses in posterior regions of mandible: one-year results from a prospective cohort study. Int J Implant Dent. 2017;3(1):43. doi: 10.1186/s40729-017-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Temizel S, Heinemann F, Dirk C, Bourauel C, Hasan I. Clinical and radiological investigations of mandibular overdentures supported by conventional or mini-dental implants: a 2-year prospective follow-up study. J Prosthet Dent. 2017;117(2):239–46.e2. doi: 10.1016/j.prosdent.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 113.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 115.Sterne JA, Hernán MA, McAleenan A, Reeves BC, Higgins JP. Cochrane handbook for systematic reviews of interventions. London: Cochrane Collaboration; 2019. Assessing risk of bias in a non-randomized study; pp. 621–41. [Google Scholar]
- 116.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beitlitum I, Sebaoun A, Nemcovsky CE, Slutzkey S. Lateral bone augmentation in narrow posterior mandibles, description of a novel approach, and analysis of results. Clin Implant Dent Relat Res. 2018;20(2):96–101. doi: 10.1111/cid.12580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. PRISMA 2020 checklist.
Data Availability Statement
Not applicable.