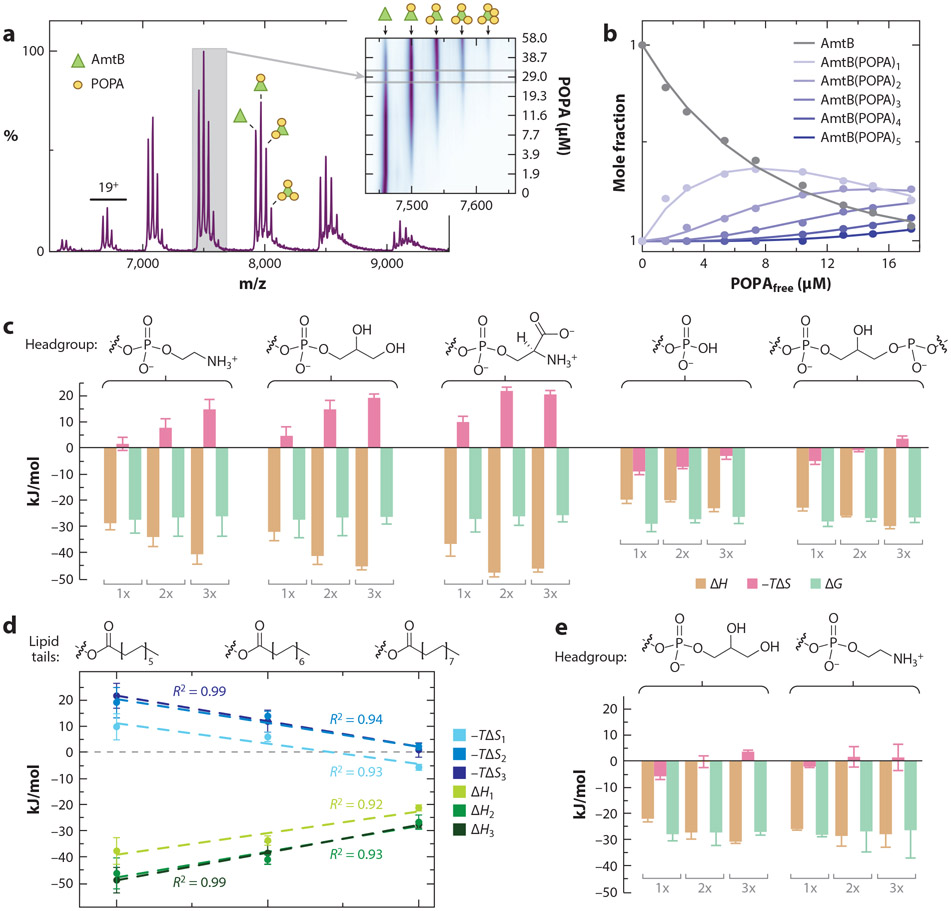
Figure 4.
Thermodynamic signatures for the interaction of lipids with the ammonia channel (AmtB), an integral membrane protein, from Escherichia coli. (a) Native mass spectrum of AmtB in the C8E4 detergent with 1-palmitoyl-2-oleoyl phosphatidic acid (POPA). The inset shows a contour plot of the 17+ charge state as functions of POPA concentration. (b) Plot of mole fraction of AmtB and lipid-bound states in the titration series (dots) and fit of a sequential lipid binding model (solid lines). (c) Thermodynamic signatures of AmtB binding phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylserine (PS), phosphatidic acid (PA) containing 1-palmitoyl-2-oleoyl (PO) tails, and 1,1′,2,2′-tetraoleoyl-cardiolipin (TOCDL) determined through van’t Hoff analysis. Binding of the first, second, and third lipid is shown as 1x, 2x, and 3x, respectively. Headgroup structures are shown above. (d) Binding thermodynamics for PG with different acyl chain length: 12 (1,2-dilauroyl), 14 (1,2-dimyristoyl), and 16 (1,2-dipalmitoyl). The first, second, and third trendlines are plotted. (e) AmtB double mutant (N72A/N79A) binding thermodynamics for POPG and POPE. Figure adapted with permission from Reference 15, copyright 2016 American Chemical Society.