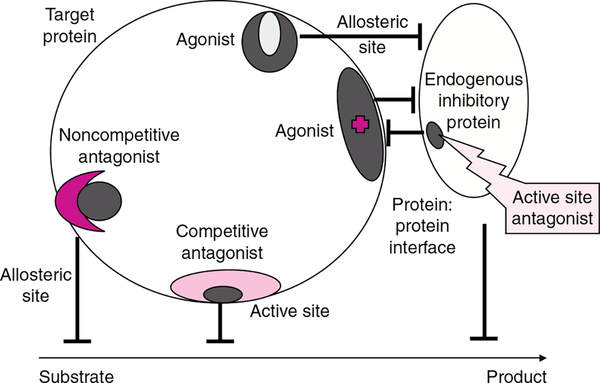
Fig. 1.
Drug discovery has frequently involved the targeting of a specific protein with small molecules that act as antagonists or agonists either at active or allosteric sites. Whether resultant drugs act competitively or non-competitively depends upon the actual site and avidity of binding. The impact on a target enzyme is usually interference with substrate-to-product conversion. More recently, the principle of interference with protein:protein interaction as a viable drug discovery approach has gained credibility. Shown in the figure is the concept of targeting an endogenous inhibitory protein (in context of this review, for example GSTP1*A). The outcome is to disassociate this protein from its partner (e.g. c-Jun N-terminal kinase) producing the agonist effect and concomitant activation of the kinase.