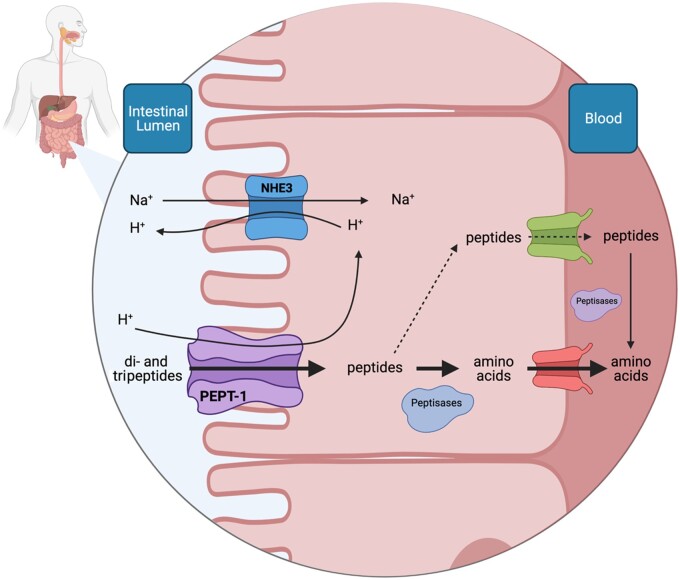
Figure 3.
Graphical representation of collagen-derived di- and tripeptide absorption from the intestinal lumen into the blood circulation. Various hydrolyzation procedures during industrial processing of collagen produce peptides, which have been proposed to have stimulatory or inhibitory properties within various tissues. Hydrolyzed collagen ingestion delivers peptides to the intestinal lumen. Collagen-derived peptides, and those containing hydroxyproline in particular, appear to be resistant to further intestinal peptide cleavage into free amino acids. These peptides may be transported through a transmembrane peptide transporter (PEPT1) into the enterocyte. Peptides transported to the cytosol of the enterocyte are either cleaved by peptidases into free amino acids and transported further into the circulation or are transported in their intact form into the circulation. Once in the circulation, peptides are subjected to cleavage through exposure to vascular endothelial tissue or peptidase activity. Overall, the existing evidence suggests that intact dietary-derived peptide uptake into the circulation is low. Whether collagen peptides, some of which are proposed to be more resistant to cleavage, have a stimulatory impact on connective tissue remodeling remains to be determined in humans