Abstract
L-asparaginase is used in chemotherapy for acute lymphoblastic leukemia and other cancers. L-asparaginase derived from bacterial source triggers immune responses. The current study investigates Solanum nigrum as a novel and latent source of L-asparaginase to minimize immunological reactions. The antitumor activity of SN methanol extract was determined using the potato disc assay. InterPro Chimera and InterPro were used to predict the amino acid sequence of L-asparaginase and its anticancer activity. Purification of the enzyme was carried out to homogeneity of 1.51-fold with a recovery of 61.99%. At optimal conditions of 36.5°C, pH 8.6, and 8.5 g/mL substrate, fruit (crude extract) revealed an L-asparaginase titer of 48.23 U/mL. The molecular weight of the enzyme was calculated to be 32 ± 5 kDa using SDS PAGE. The fruit’s total flavonoids and phenolic contents are 0.42 ± .030 g/mL and 94 ± 1.9 mg CAE, respectively. Anti-tumorigenic efficacy was determined to be 66% against Agrobacterium tumefaciens. Additionally, the extract possesses potent antifungal and antibacterial properties. Molecular docking provided the structural motifs and underlying interactions between L-asparaginase, N-acetylglucosamine, murine, and chitin. SN contains high levels of the enzyme L-asparaginase and phytochemicals, making it a potential source of anticancer drugs.
Keywords: antibacterial plant, anticancer plants, antifungal plants, flavanoids, L-asparaginase, polyphenols, Solanum nigrum
Introduction
Synthetic drugs and antibiotics cure many microbial and viral infections caused by biofilm-forming bacteria and viruses, which is of main public health concern.1,2 Allopathic medicines mostly tend to develop patentable compound “magic bullet,” which may evoke sensitivity and other allergic responses. Moreover, chemotherapeutic drugs to date also impose toxic effects on other non-target tissues. 2 In contrast, medicinal plants employed to heal the illness and restore equilibrium by converging on specific biomolecules and pathways. 2 These natural remedies have been developed overages through extensive experimentation. The frequent use of herbal extracts in local communities is due to their effective biological responses and cost-effectiveness. 3 Over 80% of the world’s population is dependent on traditional medicinal remedies for healthcare support, as they are less susceptible to side effects and more effective. 3 There are 35,000 species of plants investigated and sorted for anticancer potential like vincristine, vinblastine, and taxol, which are plant-sourced. 2 Chemotherapies do not always result in a successful and durable cure. Recently, there has been a resurgence of interest in metabolic treatment for cancer, namely amino acid depletion by enzymes. L-asparaginase has been licensed by the US Food and Drug Administration for the treatment of acute lymphoblastic leukemia. Clinical studies with arginine deaminase and recombinant human arginase as prospective cancer therapeutic agents for the treatment of arginine-auxotrophic malignancies have started. Furthermore, for the therapy of malignant leukemias, new amino acid degrading enzymes such as glutaminase, methionase, lysine oxidase, and phenylalanine ammonia lyase have been discovered. 4
Enzyme L-asparaginase (EC 3.5.1.1) (ASNase) possesses a vital role among chemotherapeutic regimens. It is administered in amalgamation with various chemotherapies like daunorubicin, cytosine arabinoside, vincristine, and L-asparaginase5,6 for different kinds of cancer such as acute lymphocytic leukemia, lymphosarcoma, melanosarcoma. ALL affected over 53,000 people in 2020 and to date ALL patients are subjected to chemotherapy. 7 L-asparaginase is the asparagine amidohydrolase, an extracellular enzyme that catalytically hydrolyzes asparagine (Asn) and liberates ammonia and aspartic acid in cancer cells. Healthy cells escape this shortage of asparagine as they recover by supplying Asn’ from L-Asparaginase synthetase. 8 Commercially, the anticancer enzyme for clinical application is obtained from Escherichia coli and Erwinia carotovora, commonly marketed as Elspar, Oncaspar, Erwinase, and Kidrolase, which induce therapeutic reactions and side effects.9-11 To avoid adverse anaphylaxis and therapeutic reactions, the anticancer enzyme L-asparaginase was elucidated from a phytomedicinal source. The members of Solanaceae family have been reported as a potent source of L-asparaginase, regarding its antiproliferative/antitumor activity against cell lines of hepatic carcinomas, colon, stomach, lung, bladder and breast cancer. 12 As far as enzyme concentration is concerned, a markedly high concentration of L-asparaginase from Vigna radiata and Tamarindus indica (Fabaceae) is also reported. 8 Solanum nigrum, 13 a phytomedicinal source well known as “Black Nightshade” and “Makoh,” 14 belongs to the genus Solanum, family Solanaceae 15 SN world widely employed for disparate ailments, including seizure and epilepsy, pain, ulcer, inflammation, diarrhea, some eye infections, and jaundice. 14 SN (fruit and leaf parts) contains flavonoids, phenolic compounds, tocopherols, polysaccharides, glycoalkaloids, and glycoproteins well-known to produce antiproliferative effects due to their immunomodulatory properties. The flavonoids and glycoalkaloids also deliver antifungal and anti-inflammatory activity by activating the proapoptotic factors or inhibiting the transcription factors, which play an essential role. 16 Numerous studies have reported that SN showed in vitro antitumor efficiency in hepatocellular carcinoma cells, human ovarian carcinoma cells, 17 human colorectal carcinoma cells, and human endometrial carcinoma cells.18,19
The commercial demand for therapeutic enzymes in cancer therapy and natural sourced antibiotics and antifungal medicines opens new windows for research. The plants are cheaper and accessible source of enzymes, yield high L-asparaginase concentration with markedly increased activity. To avoid severe hypersensitivity reactions, obtaining L-asparaginase from medicinal plants or edible sources is preferable to microbial or fungal sources.9,20 A recent study was conducted to explore L-asparaginase activity and concentration, investigate optimum milieu for the activity of L-Asparaginase from SN and its bioactivity against tumor and fungal and bacterial strains. The biological activities of SN extracts were justified and proved by molecular and mechanistic methods using bioinformatics.
Materials and Methods
Chemicals
The chemicals used in this study were L-asparagine (Sigma Aldrich), Sephadex G-100 (Sigma Aldrich Chemical Co. USA), DEAE cellulose resin (Diethylamine ethyl amine) cellulose resin, PMSF (Phenyl methyl sulfonyl fluoride), EDTA (Ethylene diamine tetra amine), SDS (Sodium dodecyl sulfate), 30% acrylamide stock (37.5: 1 acrylamide: bisacrylamide) (Bio-Rad Laboratories), TEMED (Life Technologies, Gibco®), ammonium persulfate (Sigma Aldrich), pre-stain protein MW marker (Bio-Rad Laboratories), bromophenol blue (Thermo Fisher Scientific), tris base (Calbiochem-Behring), Tris-HCl (pH 6.8), and β-mercaptoethanol (Sigma Aldrich). Fruits of SN were obtained from the botanical garden situated at the University of Agriculture, Faisalabad.
Preparation of Extracts
SN fruit (10 g) was crushed with .05 M potassium phosphate buffer (three volumes) for asparaginase extraction; pH was adjusted to 8.0 along with the addition of PMSF 1 mM, 1 mM EDTA and 10% (w/v) glycerol. The mixture of reagents and extract was centrifuged at 10,000 rpm for 20 min (SCILOGEX SCI24 Micro-Centrifuge). The next supernatant was retained as a crude enzyme.
Purification of L-Asparaginase
Ammonium sulfate precipitation was used to partially purify crude extract and dialysis against phosphate buffer at pH 8.6. Through ion-exchange chromatography consisting of a DEAE cellulose-packed column, 500 μL of the partially purified enzyme was used, and 100 fractions were collected at the rate of 30 mL/h. L-asparaginase was finally purified by gel filtration chromatography using a column filled with Sephadex G-100 resin and tris buffer at pH 8.6. The fractions collected were also analyzed spectrophotometrically at 540 nm. 8
Assay for L-asparaginase by Nesslerization
The enzymatic assay was performed using L-asparagine as substrate, and hydrolysis was undertaken by the Nessler method using phosphate buffer. The principle followed the enzymatic transformation of L-asparagine to L-aspartate, and ammonia was liberated. The ammonia concentration in samples was determined by combining .5 mL of the enzyme sample to be estimated with 4 mL of distilled water and .5 mL of Nessler’s reagent and incubating it at 37°C for 15 minutes. The absorbance was measured at a wavelength (450 nm). While protein content was determined spectrophotometrically (UV-VIS-1900, Shimadzu), crude fruit extracts and purified enzyme protein content were estimated using the Biuret method. At the same time, bovine serum albumin (BSA) was taken as a standard.18,19 The enzyme activity and the protein concentration were estimated by applying the following equations (1)-(6)
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Kinetic Characterization of Enzymes
Enzyme activity was measured at different pHs, including 5, 6, 7, 8, and 13. Different pH values were introduced by sodium acetate buffer (5 to 5.6), phosphate buffer with a pH range of 5.8 to 8.0, and borate buffer for pH (10. L-asparaginase purified from SN fruit extract was tested at various temperatures (15, 25, 30, 35, 40, 45, 50, and 55°C) and asparagine (substrate) concentrations (2 g/mL to 15 g/mL) to determine the optimal temperature and substrate amount. 7
Molecular Weight Determination
SDS PAGE was performed on 500 L of pure and lyophilized enzyme according to Laemmli et al21-23 SDS PAGE was carried out following the specifications provided by Invitrogen NuPAGE®. In brief, 7.5 μL purified enzyme (5-25 μg protein) was mixed four times with 2.5 μL LDS loading buffer (Invitrogen). After loading the sample onto precast NuPAGE Novex 12% Bis-Tris 1.0 mm mini gels (Invitrogen). Following that, each gel run was loaded with 5 μL of pre-stained SDS PAGE standards (Bio-Rad) with known molecular weights. Electrophoresis was performed at room temperature for 45 minutes at a constant voltage of 200 V in a 1X solution of NuPAGE MOPS SDS running buffer (Invitrogen) until the dye reached 60 mm of gel. Compositions of buffers were determined using the manufacturer’s technical manual. 24
Identification and Quantification of Flavonoids and Phenolic Content
Sodium Hydroxide Test for Flavonoids
A 10% sodium hydroxide aqueous solution (2 mL) was added to 5 mL of the aqueous extract filtrate from the fruit. We obtained a yellow-colored solution. After adding diluted HCL, the color changed from yellow to colorless, indicating the presence of flavonoids. 25
The crude extract of SN fruit (.5 mL) was dissolved in .1 mL of 1 M potassium acetate. An equal volume of 10% aluminum chloride was added with 1.5 mL methanol and 2.8 mL distilled water. For 30 minutes, the mixture was kept at room temperature. At 415 nm, the optical density of the mixture was determined spectrophotometrically. Rutin was used as the standard, and standard curve dilutions were prepared. The assay was performed in triplicate. The results were expressed as μgmL-1 of rutin concentration using the following calibration curve: Y = 2.142x–.1, R2 = .974, where Y indicated the absorbance and x signified the rutin concentration.15,26
Determination of Phenolic Compounds
The ferric chloride test was used to determine the presence of phenolic compounds. To a 1 mL crude extract, 5% neutral ferric chloride was added (2-3 drops). The dark green color of the solution indicated the presence of phenols. 27 To prepare the Folin Ciocalteu reagent, 700 mL distilled water was mixed with 25 g sodium molybdate, 100 g sodium tungstate, 50 mL 85% phosphoric acid, and 100 mL concentrated HCl. After adding 150 g of lithium sulfate, the total volume was 1000 mL. For 10 hours, the mixture was refluxed.
To determine the phenolic content, the gallic acid standard curve was plotted. Gallic acid was added to the Folin Ciocalteu reagent (.5 mg/4 mL aqueous Na2CO3) (1:10 in distilled water). After incubating the reaction mixtures for 30 minutes at room temperature, the absorbance at 765 nm was determined spectrophotometrically. The assay was duplicated three times. The calibration curve converted the results to gallic acid equivalents in μgmL-1.
In vitro Biological Activities
Antifungal Activity
Two fungal strains (Aspergillus niger EBL-A and Aspergillus tamari RMLC-10) 28 were grown on Sabouraud’s glucose agar medium to determine antifungal activity. At a pH of 5.3, SGA medium (glucose 40 g/L, agar 20 g/L, and peptone 10 g/L) was used to culture the fungal strains. The medium was autoclaved for 15 minutes at 121°C at a pressure of 15 psi and then transferred to autoclaved test tubes. Growth was produced in 3–4 days, as a modest modification in approach utilized by Serban et al and others.29–31 Following that, the zone of inhibition caused by the antifungal activity of SN extracts and the control fluconazole (.6 μg/ml) was measured and calculated using equation (7).
| (7) |
Antibacterial Activity
The bacterial strains used were; E coli strain (ST131) (accession number KX171170–171195). 32 Staphylococcus aureus strain 32S (ST 239) (JTJX00000000) 33 and Bacillus subtilis strain DH5α (accession number AJ004803). 34 After 15 minutes of sterilization at 121°C, the nutrient broth medium was inoculated with diluted E coli (1:1000), S aureus (1:100), and B subtilis (1:10). At 37°C, test tubes were inoculated and incubated for 12 hours. Antibacterial activity of crude extracts of SN leaf and fruit was assessed using the common well diffusion method. 31 B subtilis, S aureus, and E coli were all treated with SN leaf and fruit extracts. The results were compared to those obtained with the conventional antibiotic (Erythromycin 250 mg/L: 100 μL/30 mL). After 24 hours of incubation, the diameter of well-defined inhibition zones was determined, indicating antimicrobial activity, and the percent inhibition was calculated 35 (Table 1) (Figure 3).
Table 1.
Antifungal activity of Solanum nigrum extracts against fungal strains of Aspergillus niger and Aspergillus tamari; antibacterial activity of SN extract (10μg/mL) against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis
|
Plant Part |
Fungal strains/Bacterial strain | Control Growth (mm) | Treatment Growth in mm (Mean SD) | Inhibition of Bacterial/Fungal Growth (%) |
|---|---|---|---|---|
| Fruit | Aspergillus niger | 65 | 34 ± .00 | 52.30 |
| Fruit | Aspergillus Tamari | 55 | 30 ± .05 | 54.54 |
| Fruit | Escherichia coli | 15 | 11.95 ± 1.5 | 42.61 |
| Fruit | Staphylococcus aureus | 13.55 | 12.6 ± .32 | 34.39 |
| Fruit | Bacillus subtilis | 13.4 | 11.8 ± 1.43 | 31.33 |
Figure 3.
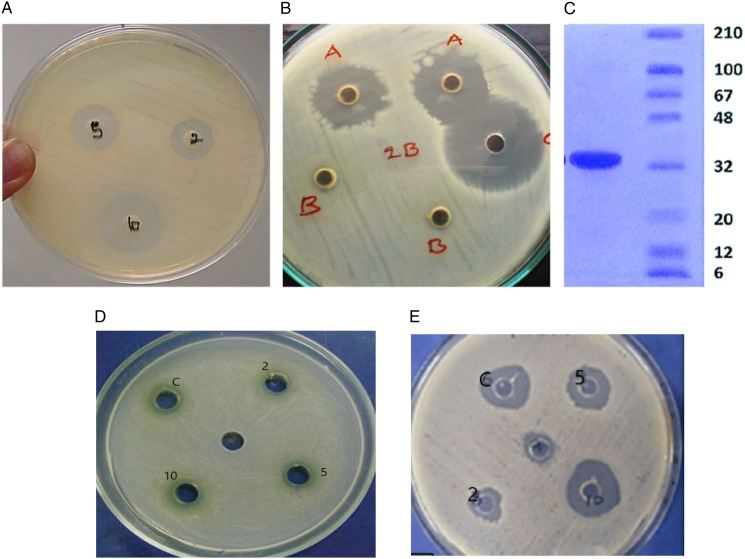
(A) Antibacterial assay, regarding Solanum nigrum extract inhibiting the bacterial lawn of B. subtilus (B) antifungal assay, the effect of Solanum nigrum extract on the fungal growth of Aspergillus tamari (C) SDS PAGE depicted molecular weight of S nigrum (32 ± .5 kDa) as compared to the standard proteins, with molecular weights ranging from 6 kDa to 210 kDa. (D) E. coli growth inhibited maximal by 10 g/mL concentration of SN fruit ext. (E) Staphylococcus aureus zones of inhibition by fruit extract of SN as compared to erythromycin control.
Antitumor Activity
The antitumor activity of SN methanol extract was determined using the potato disc assay. On Luria Bertani (LB) agar medium, an A tumefaciens strain was cultured.36,37 Sterilized LB broth medium containing phosphate buffer pH 7.3. Cork-borers were used to cut potatoes obtained from the market into 5 × 8 mm sections. Discs were placed in agar medium in Petri plates (10 discs per plate). Petri plates were inoculated with a sufficient amount of 50 μL of A tumefaciens inoculums, and plant extract (leaves) was incubated at 27 ± 3°C for 2 days. To determine the antitumor activity of the SN extract, 600 μL of plant extract were diluted with 150 μL of sterilized distilled water and combined with 800 μL A tumefaciens in PBS.
The mixture was injected into potato disc galls. Standard Camptothecin 30 ppm (1 ppm = 1 mg/L) was used as a control for extract activity on potato discs. After four weeks of incubation at 28–30°C, potato discs were stained with Lugol’s solutions (10% KI, 5% I2). Gall tumors were counted under a microscope to determine the tumor percentage. 38
In silico Anticancer Activity
L-asparaginase’s antifungal and anticancer activity was confirmed using the bioinformatics tools Chimera and InterPro Scan. Docking was performed using Chimera 1.13.1. 39 To ascertain the enzyme L-asparaginase anticancer activity, the amino acid sequence was functionally predicted using InterPro Scan, and the amino acid sequence was retrieved from the NCBI protein repository (Mitchell et al., 2019).
Statistical Analysis
The analyses were conducted in triplicate, mean and standard deviation was calculated (mean ± SD). The observations were expressed both in absolute numbers and in terms of the standard error of the mean by using SPSS version 21. The two extracts’ percentage inhibition of cell growth was determined using their respective absorbance values at all concentrations. 2
Results
Purification of L-Asparaginase
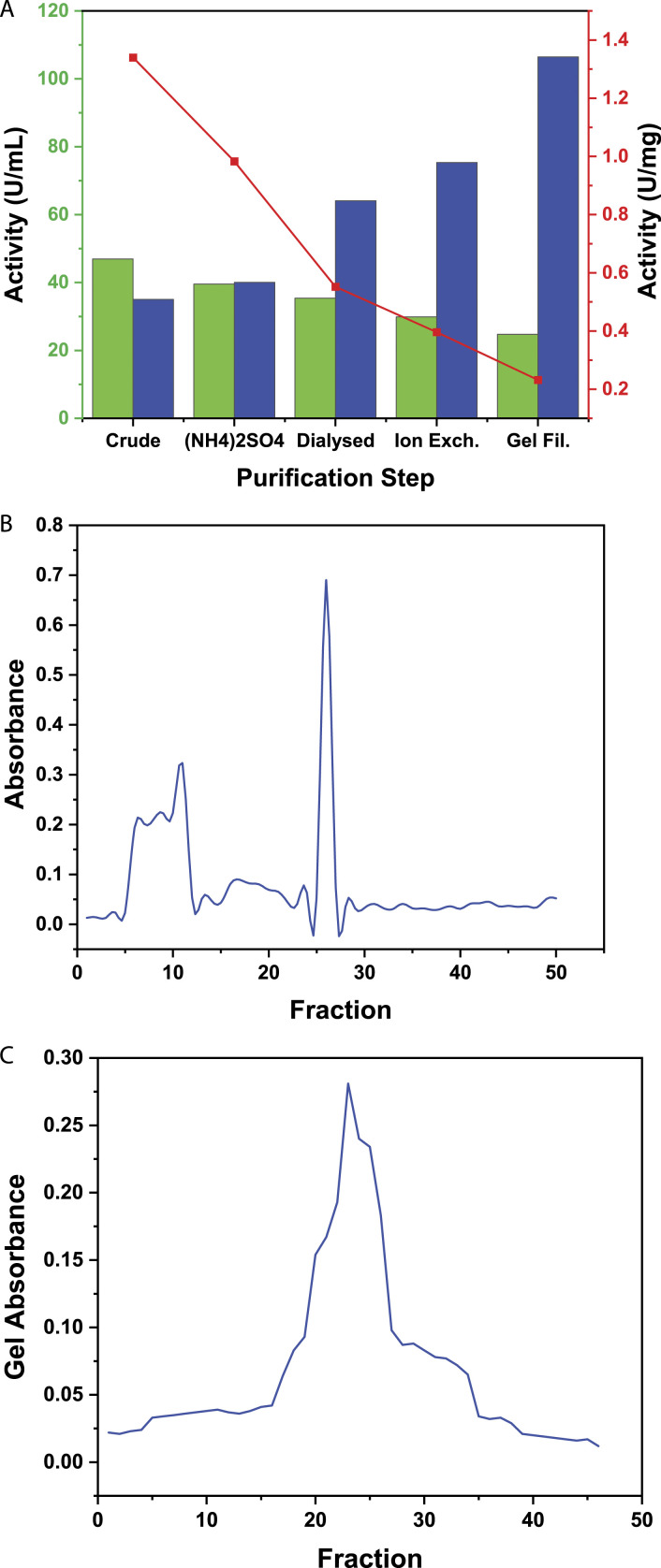
The enzyme purification steps are depicted in Figure 1A. After sorting the ion-exchange chromatography fractions9,10,25,26 containing a high concentration of protein and enzyme, 500 μL of the eluent from these fractions was introduced to gel filtration process, while utilizing Sephadex G-100 more than fifty fractions were collected, out of which numerous fractions revealed peaks like in fraction number 22, 23 with the absorbance .278 and .265 as shown in Figure 1C.
Figure 1.
The experimental results of (A) enzyme purifications, (B) ion exchange, and (C) gel filtration of L-asparaginase from Solanum nigrum.
The crude extract of SN fruit contained 64.38 U/mL L-asparaginase activity, whereas the purified enzyme received in the 24th fraction after gel filtration contained 43.98 U/mL activity and 95.67 U/mg specific activity. The crude extract of SN contained 66.27 U/mL L-asparaginase activity and 1.02 mg protein, which gradually decreased with each purification step. It eventually revealed a concentration of 43.98 U/mL and protein content of .451 mg. While the specific activity increased from 64.38 U/mg crude enzymes to 95.67 U/mg purified enzyme as illustrated in Figure 1B.
Characterization of Enzyme
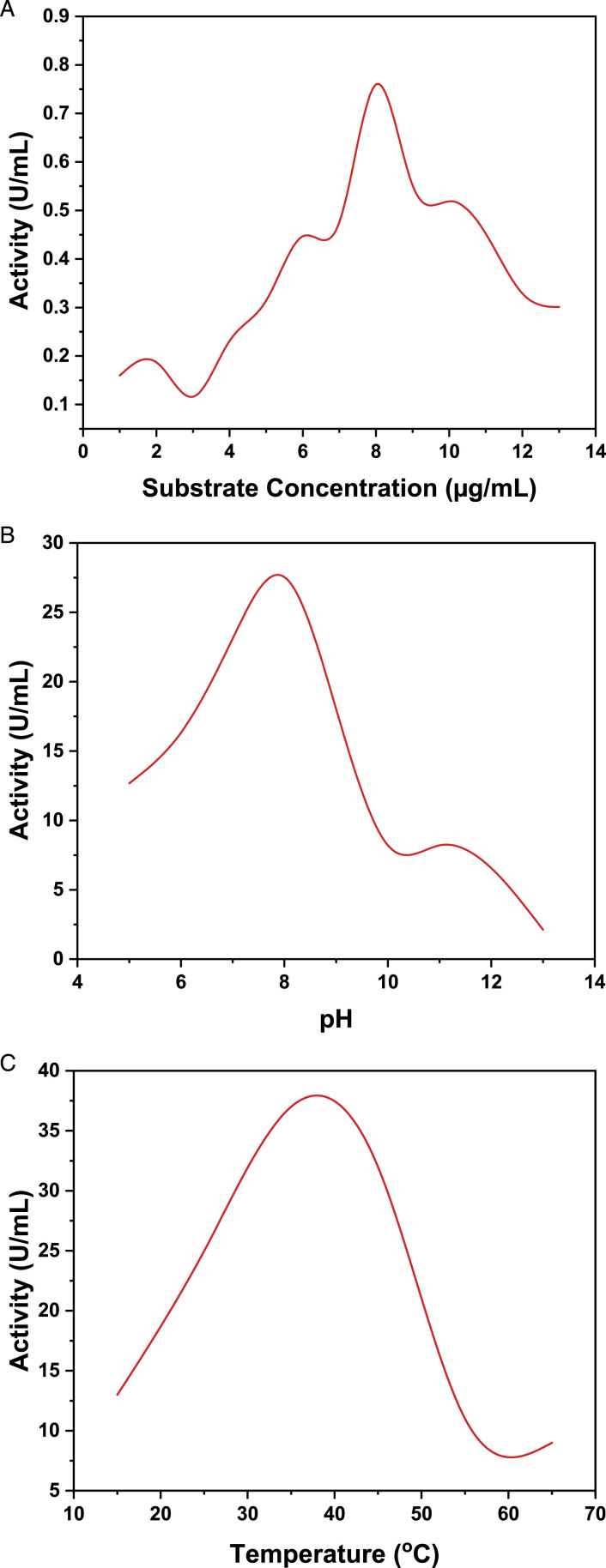
The enzyme was purified from the fruit of SN. The optimal enzyme activity was observed at a pH of 8.4 and a temperature of 36.5°C in the presence of .2 M phosphate buffer (see Figure 2B and C). The peak activity of L-asparaginase was observed at 8.5 g/mL substrate concentration (see Figure 2A). At pH 8.4, the activity reached a maximum of 27.54 U/mL, while .76 U/mL activity was obtained when 8.5 g/mL L-asparagine amino acid substrate was consumed (see Figure 2A).
Figure 2.
Effect of (A) substrate concentration, (B) pH, and (C) temperature on the activity of L-asparaginase from Solanum nigrum.
The results indicated that the plant extract (fruit) possessed a broad spectrum of antibacterial activity, as evidenced by the formation of distinct inhibition regions in bacterial lawns of various strains. In comparison to 2 g/mL and 5 g/mL, the concentration of 10 g/mL exhibited the largest zone of inhibition (Figure 3A, B, D and E). The growth of E coli, S aureus and B. Subtilis observed were 11.95 ± 1.5, 12.6 ± .32, 11.8 ± 1.43 nm, respectively, against the 10 g/mL concentration of plant fruit extract. Yet the MIC was observed to be 2 mg/dL for B. Subtilis and 5 mg/d for E coli and S aureus. The antifungal activity of methanol extracts of SN fruit was determined using the Well diffusion method following 3–4 days of Aspergillus growth on Sabraud medium in petri plates. S nigrum fruit (10 g/mL) extract inhibited maximum fungal growth of A niger and A tamari by 52.03 and 54.54%, with the growth zones of 34 ± .00 and 30 ± .05 nm, respectively (Table 1). The MIC of SN was recorded to be 2 g/mL for fungal growth retardation.
For testing enzyme purity, SDS PAGE was employed. The molecular weight of SN has been determined to be 32 ± 5 kDa (Figure 3C).
Antitumor Activity
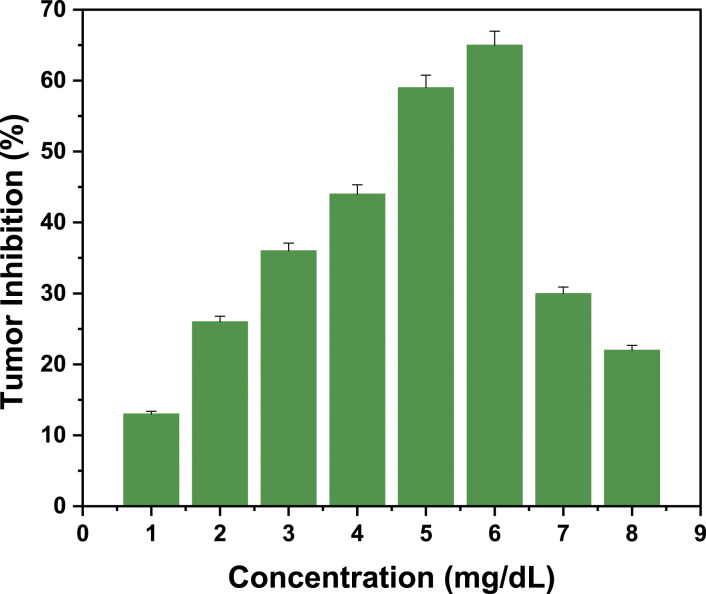
Antitumor activity was determined using the A tumefaciens strain. The sensitivity of gall tumors of A tumefaciens against SN leaf extract was used as a cursor for antitumor activity. The control potato discs had developed a few millimeters of proliferative tissue 3-4 weeks after inoculation. The test samples of potato discs had developed crown gall tumors, as indicated by the percentage inhibition in Figure 4. The maximum inhibition of 65%, was secured by the 6 mg/dL concentration of S.N fruit extract.
Figure 4.
Antitumor activity (Potato disc assay) of methanolic extracts of Solanum nigrum on (Agrobacterium tumefaciens) strain AtSl0105 against Campothacin (30ppm) as control.
Bioinformatic Confirmation of the Anticancer and Antibacterial Potential of SN
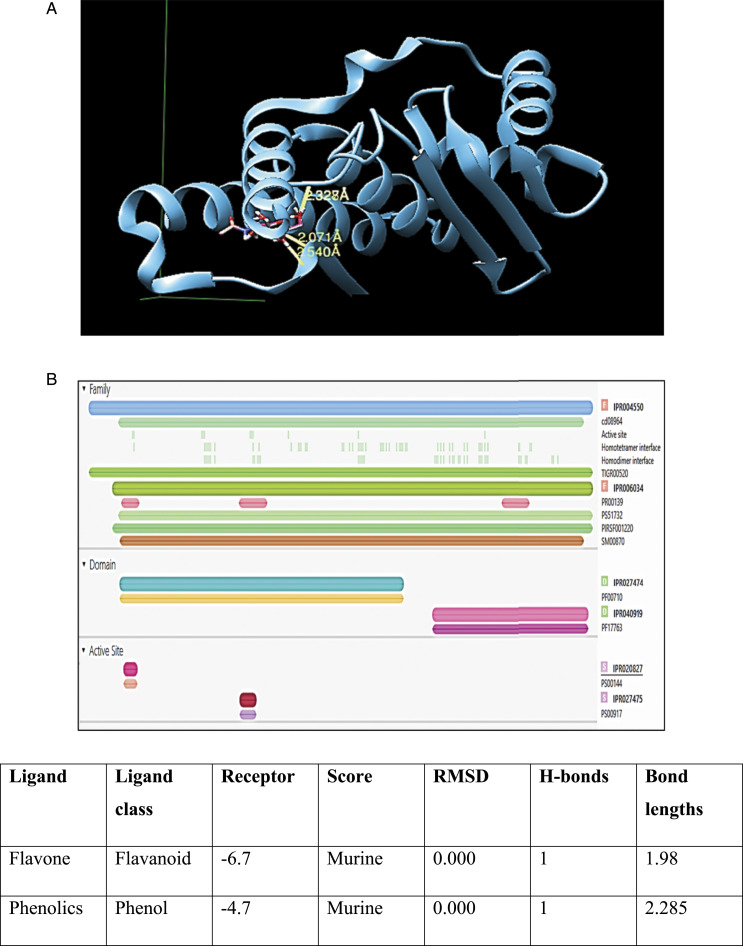
Chimera docking revealed sufficient interactions between chitin and L-asparaginase with bond lengths of 2.32, 2.07, and 2.54 Å with RMSD value .00 and score −5.5 (see Figure 5A). InterPro Scan established the anticancer activity of L-asparaginase by presenting its domain IPR027474 and active sites IPR020827 and IPR027475. These active sites catalyze the deamination of L-asparagine, which results in the death of tumor cells (see Figure 5B).
Figure 5.
(A) Docking of L-asparaginase with Chitin monomer 2-acetamido-2-deoxy-beta-D-glucopyranose. (B) Functional analysis of L-asparaginase to validate its anti-cancerous activity. (C) The bactericidal potential of flavonoids and phenols; Flavonoids and phenolics (phytochemicals) interaction with a murine component of bacterial cell wall ending up cell death. Both flavonoids and phenols offer a 1 h bond with murine (a component of the bacterial cell wall), with bond lengths 1.98 and 2.285, respectively.
Figure 5A illustrates the results of L-asparaginase and chitin docking. The optimal results had the highest number of hydrogen bonds, the lowest (energy score), and an RMSD value close to zero. Three hydrogen bonds with bond lengths of 2.32, 2.07, and 2.54 Å were optimal docking results. The RMSD value was .00, and the score was −5.5. The results were satisfactory and indicated proper bonding between L-asparaginase and chitin monomer N-acetylglucosamine.
L-asparaginase has the IPR027474 domain and the active sites IPR020827 and IPR027475. The domain and active sites catalyze the deamination of L-asparagine. The illustration of functional analysis is presented in Figure 5B.
Additionally, SN may help alleviate the mucositis associated with chemotherapy.12,40 In Chinese medicinal plants, SN’s diuretic and antipyretic properties aid in treating a variety of alleviated inflammations such as edema. 41
Mechanism Involved in Apoptosis of Cancer Cells by Flavonoids and Phenolic Compounds
In crude methanolic extracts of SN, flavonoids and phenolic compounds were identified qualitatively. Quantification of flavonoids and phenols was performed following the methodology described in the section Identification and Quantification of Flavonoids and Phenolic Content. The concentration of flavonoids in the SN (fruit) extract was .42 ± .030μg mL-1, equivalent to 94 ± 1.9 mg chlorogenic acid (mg CAE).
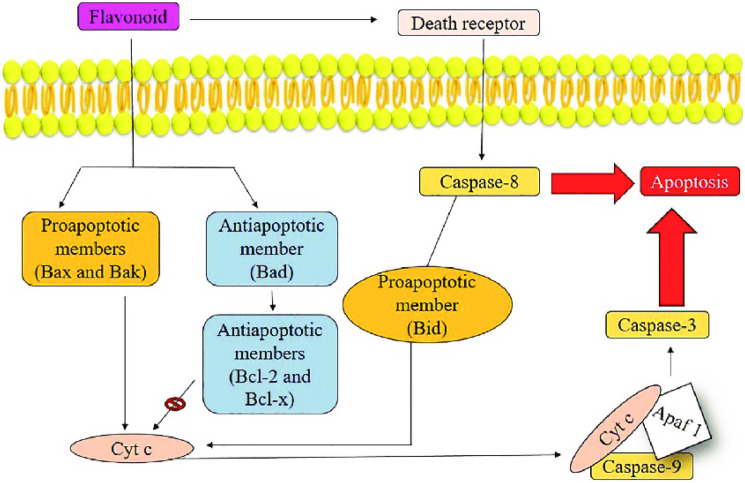
Flavonoids participate in the extrinsic apoptotic pathway by activating apoptotic proteins and transcription factors, resulting in cancer cell apoptosis. The apoptotic complex (apoptosome) is composed of caspase-8, which activates caspase-3 and cytochrome C (a component of the electron transport chain) in the mitochondrion, transmitting numerous signals for cell death via the extrinsic pathway. Caspase-9 also activates caspase-3, which cleaves target proapoptotic family proteins (e.g., Bax, Bad, Bid, and Bak) and anti-apoptotic family proteins (e.g., BCL-2, McL-1, and Bcl-xL) that are primarily responsible for cell death (Figure 6).
Figure 6.
Solanum nigrum extracts ameliorating apoptotic cascade of cancer/tumor cells by Flavonoids; Bax, Bak, and Bid are regulators of apoptosis. BcL-x, Bad, and BcL-2 are regulatory proteins for apoptosis cascade (inhibited by flavonoids), procaspase-9 and Apaf1 and cytochrome C combine to form apoptosome. Simultaneously, death receptors integrate with procaspase-8, triggering caspase-3 and activating apoptosis. 53
Discussion
Southern Indian people use the leaves of the SN plant as herbal medicine in traditional systems to treat mouth ulcers, constipation, arthritis (rheumatism), and even as an anticancer. 41 Kaur and colleagues discovered that SN leaves have the highest enzymatic activity (52 U/mL), followed by fruit (48 U/mL), and other plant parts,11,42,43 close to 64.38 U/mL determined in this study from SN fruit extracts.
The purified enzyme was subjected to a series of tests to determine the optimal conditions for its activity and asparagine specificity (used as substrate). The concentration of the substrate affects the rate of the reaction (enzyme activity) until optimal conditions are reached. However, enzyme saturation limits both the rate of the reaction and the concentration of the substrate. The enzyme potency was maintained constant in the laboratory, while the substrate concentration was gradually increased until the optima was achieved for enzymatic activity. Similarly, temperature and pH parameters behaved optimally in a specific manner. Likewise, L-asparaginase derived from Pisum sativum from same family of plants, exhibited maximum activity at 37°C, and phosphate buffer was a suitable buffer for enzymatic stability. 44 The current study established that L-asparaginase retained efficacy at pH values ranging from 5.0 to 9.0 and temperatures ranging from 40 to 45°C, as previously reported. 13
The extracted and purified enzyme L-asparaginase appeared to have a molecular weight of 32 kDa, comparable to the molecular weight of beans (P. Vulgaris) from the Fabaceae family, which consists of homodimer subunits of 40.6 kDa. 45 While the molecular weight of L-asparaginase extracted from Withania somnifera fruit was determined to be 72 ±.5 kDa, 46 this indicates that the molecular weight of the L-asparaginase molecule varies even within plants. Pharmaceutical compounds produced from plants are a viable strategy for drug discovery. Polyphenols, a class of phytochemicals that includes phenolic acid, flavonoids, and tannins, exhibit substantial radical scavenging effects, for preserving the viability of cells and cell membranes by reacting with reactive oxygen species.15,47,48 Among polyphenols, flavanoids are well-known to have anticancer, antibacterial, and fungicidal properties (Figures 5 and 6). Campisi reported a significant concentration of total polyphenolic content and flavone components in SN (leaf extract), which improved the oxidative state of primary cultures of astrocytes 14
Microbial infections are widely recognized as the leading cause of morbidity and mortality. Using the well diffusion method, the antibacterial activity was observed as a distinct zone of inhibition. A fruit extract at a concentration of 10 g/mL inhibited the growth of both Gram-positive (B subtillus, S aureus) and Gram-negative bacteria (E coli). 49 These findings corroborate those of Mahmood et al, 50 who investigated the antimicrobial activity of L-Asparaginase isolated from Datura Inoxia against the list of microbes used in this work. The antifungal activity of S nigrum (fruit) is remarkable. Crude plant extracts showed that the SN methanolic extract is packed with the biologically active components of plant parts (see Table 1). The ethanol extract of R. longipes demonstrated broad-spectrum antibacterial activity with inhibition zone of 25.5 mm against S aureus, 27.5 mm against E coli. 51 While SN fruit extract had established 34.39 nm zone against S aureus and 45.61 nm against E coli.
The fruit exhibited a moderate level of antifungal activity. The antifungal activity of the fruit was 54.54% inhibitory against A tamari. Surrattense Solanum bacteriostatic and fungicidal activity is attributed to recently discovered steroidal alkaloids and glycosides.19,52 Nitric oxide (NO)-induced apoptosis in fungal and bacterial lawns is induced by lipopolysaccharide (LPS). Additionally, these bioactive compounds aid plants in defending themselves against bacterial and pest invasion. 14
The anticancer assay was carried out using the potato discs technique in the same manner as performed by Mahmood et al. 50 The control group’s gall formation was compared to the results of the plant extract treatments.
In the current study, tumors induced by A tumefaciens were treated with eight plant extracts ranging from 1 mg/dL to 8 mg/dL. The tumor reduction was determined using the plant extracts' concentration gradient compared to negative control of potato discs. The highest antitumor activity (65%) was obtained at a concentration of 6 mg/dL. Flavonoids and polyphenols also play a significant role in tumor cell apoptosis. The fruit contains flavonoids and phenolic acids in concentrations of .42 ±.030 μg mL–1 and 94 ±1.9 mg (mg CAE), respectively, which act as scavengers of reactive oxygen species (ROS). ROS cause DNA damage, which results in mutations, tumorgenesis, and angiogenesis. Flavonoids have the potential to inhibit angiogenesis. Endothelial cell migration, lumen formation, and proliferation are all stages of the angiogenesis cascade. Flavonoids are well-known inhibitors that play a critical role in interfering with angiogenesis steps. In response to flavonoids and phenols, the proteins promote the formation and activation of apoptosomes, which disintegrates cellular components, the rupture of the cell membrane, and ultimately the death of tumor cells. Another mechanism by which flavonoids kill tumor cells is inhibiting protein kinases involved in the angiogenesis signal transduction cascade. 53
The chosen docking results had the lowest energy score, an RMSD value close to zero, and the maximum number of hydrogen bonds. Chitin is the primary component of the fungal cell wall. N-acetylglucosamine, a chitin monomer, was chosen for docking with L-asparaginase to demonstrate the antifungal activity of L-asparaginase. L-asparaginase forms hydrogen bonds with Chitin monomers, weakening and eventually killing the fungal cell wall. This bonding impairs the cell wall and ultimately destroys the pathogen cell. In cancerous cells, this process is irreversible and results in apoptosis. L-asparaginase has antioxidant properties and is considered an anti-cancerous, antiproliferative biomolecule. 20 SN leaves contain significant amounts of calcium, iron, phosphorus, and vitamins A and C. Vitamin C, as an antioxidant, has been shown to kill cancer cells by generating intracellular H2O2, a cell death signal.54,55
Function prediction was performed to substantiate a claim about SN’s anticancer potential. The domain and active sites catalyze the deamination of L-asparagine to produce aspartic acid and an ammonium ion. 56 Functional analysis for L-asparaginase shows that it converts asparagine to aspartic acid. 57 Cancerous cells are deficient in Asparagine synthetase. Thus, once asparagine has been converted to aspartic acid, it cannot be reversed, resulting in asparagine depletion and eventual cell death. 58 In rat models, the protective effect of SN aqueous extract in the treatment of oral mucositis has been established. 12 Cytotoxic activity of the plant compounds against liver cancer cell line (HepG2), breast cancer cell line (MCF-7) and a human melanocyte (normal cell line) (HFB-4) in vitro were reported recently by. 59 Penicillin-binding protein 2a (PBP2a) was revealed as target that mediates both for the antibacterial and the antibiotic-synergistic consequences of phenolics. Further molecular docking and molecular dynamic simulation experiments proved that phenolics have potential antibacterial effects against the methicillin-resistant S aureus (MRSA). 60
The antiproliferative activity of the crude and extracted bioactive compounds of SN was also evaluated on a variety of tumor cell lines, including breast cancer (MCF-7), 61 Ehrlich ascites carcinoma cell (EACC) line and Hepatoma cell (HepG2) line, 62 colon cancer (HT 29), 16 and cervical cancer (U14 and HeLa), 63 indicating that SN is an excellent candidate for therapeutic regimens designed in chemotherapy.
Acknowledgments
The authors wish to express their gratitude to the Institute of Physiology and Pharmacology at the University of Agriculture, Faisalabad, for providing laboratory equipment and supplies. We would like to express our appreciation to the Department of Microbiology at the University of Agriculture in Faisalabad, Pakistan, for their kind donation of microbial strains.
Abbreviations
- SN
Solanum Nigrum
- ADR
Adverse drug reaction
- ALL
Acute lymphoblastic leukemia
- AST
antibiotic susceptibility testing
- DEAE
diethyl ethanolamine
- EDTA
ethylene diamine tetra amine
- MIC
minimum inhibitory concentration
- PBS
para benzoic sulfate
- PMSF
phenylmethylsulfonyl fluoride
- SDS PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Footnotes
Author Contributions: Dr. Ambreen Aisha performed the enzyme analysis part and wrote the main article; Dr Saba Zahra and Dr Alishbah Roobi assisted with data analysis antitumor assay; Asim Hussain and Dr Nadia Afsheen performed antimicrobial assay; Dr Naheed Bano assisted in the validation process. Dr Yasir Saleem formated and revised the manuscript. Dr Imtiaz Mahmood Tahir presented the primary hypothesis, and later reviewed, revised the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conclusion
Natural agents may be used to combat cancer’s hallmarks; S nigrum provided a scientific rationale for its antitumor, bacteriostatic, and fungicidal activity. The in vitro findings of methanolic extract of SN fruit are established through simulations to justify as a potential source of antifungal and antibiotic drugs. The SN molecular complexity, inherent bioactivity against microbes, affordability, ease of availability, lack of significant toxic effects, and high concentration of antitumor enzyme L-Asparaginase define its suitability for treating premalignant neoplasms inhibiting tumor progression. Stability studies on the enzyme’s activity indicated that it is stable up to 45°C and retains activity over a broad pH range (5.0-9.0). The antitumor activity of methanolic extracts (6-8 mg/dL) of SN was 65% in A tumefaciens. The MIC of fruit extract was 2 g/mL against bacterial growths while maximum clear zones were observed with 10 g/mL. Flavonoids and phenolics (phytochemicals) interaction with a murine component of bacterial cell wall and Chitin monomer 2-acetamido-2-deoxy-beta-D-glucopyranose prove the effective interaction with murine and chitin, respectively. Additional validation studies are necessary to ascertain the extracts' cytotoxicity and the optimal dosage of therapeutic formulations.
ORCID iDs
Ambreen Aisha https://orcid.org/0000-0002-8006-0307
Saba Zahra https://orcid.org/0000-0002-2307-0218
References
- 1.Ingle AP, Wagh S, Biswas J, Mondal M, Feitosa CM, Rai M. Phyto-fabrication of different nanoparticles and evaluation of their antibacterial and anti-biofilm efficacy. Curr Nanosci. 2020;16(6):1002-1015. [Google Scholar]
- 2.Nyaga JM, Mukavi JW, Mayeku PW, Kituyi SN. In vitro anti-cancer efficacy and phyto-chemical screening of solvent extracts of Kigelia Africana. Lam.) Benth. Heliyon 2021;6(7):e04481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Wyk B-E, Wink M. Medicinal Plants of the World. Wallingford, UK: CABI; 2018. [Google Scholar]
- 4.Wang Z, Xie Q, Zhou H, Zhang M, Shen J, Ju D. Amino acid degrading enzymes and autophagy in cancer therapy. Front Pharmacol. 2021;11:582587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J-J, Liao AT, Wang S-L. L-Asparaginase, doxorubicin, vincristine, and prednisolone (LHOP) chemotherapy as a first-line treatment for dogs with multicentric lymphoma. Animals. 2021;11(8):2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munir N, Zia MA, Sharif S, et al. L-Asparaginase potential in acrylamide mitigation from foodstuff: a mini-review. Prog Nutr. 2019;21(3):498-506. [Google Scholar]
- 7.Moharib SA. Anticancer activity of L-asparaginase produced from vigna unguiculata. World Sci Res. 2018;5(1):1-12. [Google Scholar]
- 8.Aisha A, Zia MA, ASGER M, Muhammad F. L-asparaginase, acrylamide quenching enzyme production from leaves of Tamarindus indica and seeds of Vigna radiata–Fabaceae. Pakistan J Bot. 2020;1(1):243-249. [Google Scholar]
- 9.Pui C-H. Is Erwinase necessary for all children with ALL and allergic reactions to E coli asparaginase? Pediatr Blood Cancer. 2016;63(4):587-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effer B, Kleingesinds EK, Lima GM, et al. Glycosylation of erwinase results in active protein less recognized by antibodies. Biochem Eng J. 2020;163:107750. [Google Scholar]
- 11.Kataria M, Kaur N, Narula R, Kumar K, Kataria S, Verma N. L-Asparaginase from novel source-Solanum nigrum and development of asparagine biosensor. Pharma Innov. 2015;4(5):81. [Google Scholar]
- 12.Patel A, Biswas S, Shoja MH, Ramalingayya GV, Nandakumar K. Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis. Sci World J. 2014;2014:345939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanmugaprakash M, Jayashree C, Vinothkumar V, et al. Biochemical characterization and antitumor activity of three phase partitioned L-asparaginase from Capsicum annuum L. Sep Purif Technol. 2015;142:258-267. [Google Scholar]
- 14.Campisi A, Acquaviva R, Raciti G, Duro A, Rizzo M, Santagati NA. Antioxidant activities of Solanum nigrum L. leaf extracts determined in in vitro cellular models. Foods. 2019;8(2):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aisha A, Arshad A, Goher S, Iqbal M. Bactericidal, antioxidant activity and In silico analysis of phytochemicals derived from selected plants of solanaceae family. Am Int J Biol Life Sci. 2020;2(1):28-41. [Google Scholar]
- 16.Gabrani R, Jain R, Sharma A, Sarethy IP, Dang S, Gupta S. Antiproliferative effect of Solanum nigrum on human leukemic cell lines. Indian J Pharm Sci. 2012;74(5):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C-K, Lin Y-F, Tai C-J, et al. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin-and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells. Evid Based Complement Alternat Med. 2015;2015:675270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai C-J, Wang C-K, Chang Y-J, Lin C-S, Tai C-J. Aqueous extract of Solanum nigrum leaf activates autophagic cell death and enhances docetaxel-induced cytotoxicity in human endometrial carcinoma cells. Evid Based Complement Alternat Med. 2012;2012:859185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu X-Y, Shen X-F, Wang L, et al. Bioactive steroidal alkaloids from the fruits of Solanum nigrum. Phytochemistry. 2018;147:125-131. [DOI] [PubMed] [Google Scholar]
- 20.Moharib SA. Anticancer activity of L-asparaginase produced from Vigna unguiculata. World Sci Res. 2018;5(1):1-12. [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-685. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc. 2009;4(10):1513-1521. [DOI] [PubMed] [Google Scholar]
- 23.He F. Laemmli-sds-page. Bio-Protocol. 2011;1(11):e80. [Google Scholar]
- 24.Nowakowski AB, Wobig WJ, Petering DH. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics. 2014;6(5):1068-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latif A, Ashiq K, Qayyum M, Ashiq S, Ali E, Anwer I. Phytochemical and pharmacological profile of the medicinal herb: Bryophyllum pinnatum. Journal Anim Plant Sci. 2019;29(6):1528-1534. [Google Scholar]
- 26.Amir M, Khan A, Mujeeb M, Ahmad MA, Siddiqui NA. Phytochemical screening and in vitro antioxidant activity of Jawarish Amla-A poly herbal formulation. Phcog J. 2011;3(26):54-60. [Google Scholar]
- 27.Ben I, Woode E, Abotsi W, Boakye-Gyasi E. Preliminary phytochemical screening and in vitro antioxidant prop-erties of Trichilia monadelpha (Thonn.) JJ De Wilde (Meliaceae). J Med Biomed Sci. 2013;2(2):6-15. [Google Scholar]
- 28.Munir M, Abdullah R, Ul Haq I, Kaleem A, Iqtedar M, Naz S. Strain improvement by random mutagenesis of Aspergillus Tamarii RMLC-10 for improved biosynthesis of polygalacturonase. Pak J Bot. 2020;52:1809-1813. [Google Scholar]
- 29.Cruickshank R. Medical Microbiology: The Practice of Medical Microbiology. London, UK: Churchill Livingstone; 1975. [Google Scholar]
- 30.Jamil A, Shahid M, Khan M, Ashraf M. Screening of some medicinal plants for isolation of antifungal proteins and peptides. Pak J Bot. 2007;39(1):211-221. [Google Scholar]
- 31.Şerban ES, Ionescu M, Matinca D, Maier CS, Bojiţă MT. Screening of the antibacterial and antifungal activity of eight volatile essential oils. FARMACIA. 2011;59(3):440-446. [Google Scholar]
- 32.Ali I, Rafaque Z, Ahmed I, et al. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC Infect Dis. 2019;19(1):620-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan S, Sung K, Iram S, Nawaz M, Xu J, Marasa B. Draft genome sequences of two methicillin-resistant clinical Staphylococcus aureus isolates. Genome Announc. 2016;4(1):e01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soldo B, Lazarevic V, Karamata D. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168a. Microbiology. 2002;148(7):2079-2087. [DOI] [PubMed] [Google Scholar]
- 35.Qasim N, Shahid M, Yousaf F, et al. Therapeutic potential of selected varieties of phoenix dactylifera L. against microbial biofilm and free radical damage to DNA. Dose-Response. 2020;18(4):1559325820962609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannan MA, Sarker TC, Kabir AH, Rahman M, Alam MF. Antitumor properties of two traditional aromatic rice genotypes (Kalijira and Chinigura). Avicenna J phytomedicine. 2014;4(1):31-42. [PMC free article] [PubMed] [Google Scholar]
- 37.Noor N, Sarfraz RA, Ali S, Shahid M. Antitumour and antioxidant potential of some selected Pakistani honeys. Food Chem. 2014;143:362-366. [DOI] [PubMed] [Google Scholar]
- 38.Mclaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf J. 1998;32(2):513-524. [Google Scholar]
- 39.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605-1612. [DOI] [PubMed] [Google Scholar]
- 40.Shah VV, Shah ND, Patrekar PV. Medicinal plants from Solanaceae family. Res J Pharm Technol. 2013;6(2):143-151. [Google Scholar]
- 41.Aires A, Marrinhas E, Carvalho R, Dias C, Saavedra MJ. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res Int. 2016;2016:5201879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathak N, Prajneshu M, Shaban Ahmad LK, Bhaduri A, Dhandapani A, Sharma O. Phytochemical analysis and antifungal activity of weed extracts against Rhizoctonia root rot in buckwheat (Fagopyrum Tataricum). Biopestic Int. 2021. 16(2):125-131. [Google Scholar]
- 43.Pathak T, Kumar R, Kaur J, Kumar K. Isolation of L-Asparaginase from Cannabis sativa and development of biosensor for detection of Asparagine in leukemic serum samples. Res J Pharm Technol. 2014;7(8):850-855. [Google Scholar]
- 44.Khalaf ZA, Al-Ani NK, Jasim HM. Optimum conditions for asparaginase extraction from Pisum sativum subspp Jof. Iran J Plant Physiol. 2012;2:517-521. [Google Scholar]
- 45.Al Zobaidy H, Shakir KA, Strasburg G. Characterization of l-asparaginase purified from pole beans. Iraqi J Agric Sci. 2016;47:129-137. [Google Scholar]
- 46.Oza VP, Parmar PP, Kumar S, Subramanian RB. Anticancer properties of highly purified L-asparaginase from Withania somnifera L. against acute lymphoblastic leukemia. Appl Biochem Biotechnol. 2010;160(6):1833-1840. [DOI] [PubMed] [Google Scholar]
- 47.Riaz M, Mahmood Z, Shahid M, et al. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int J Immunopathol Pharmacol. 2016;29(3):421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riaz M, Shahid M, Jamil A, Saqib M. In vitro antioxidant potential of selected aphrodisiac medicinal plants. J Biol Regul Homeost Agents. 2017;31(2):419-424. [PubMed] [Google Scholar]
- 49.Hegazy WK, Moharam ME. L-asparaginase hyperproducing recombinant Bacillus strains obtained by interspecific protoplast fusion. J Genet Eng Biotechnol. 2010;8(2):67-74. [Google Scholar]
- 50.Mahmood A, Mahmood A, Mahmood M. In vitro biological activities of most common medicinal plants of family Solanaceae. World Appl Sci J. 2012;17(8):1026-1032. [Google Scholar]
- 51.Olasunkanmi AA, Fadahunsi OS, Adegbola PI. Gas chromatography-Mass spectroscopic, high performance liquid chromatographic and In-silico characterization of antimicrobial and antioxidant constituents of Rhus longipes (Engl). Arab J Chem. 2022;15(2):103601. [Google Scholar]
- 52.Xiang L, Wang Y, Yi X, He X. Anti-inflammatory steroidal glycosides from the berries of Solanum nigrum L.(European black nightshade). Phytochemistry. 2018;148:87-96. [DOI] [PubMed] [Google Scholar]
- 53.Duraipandiyan V, Raja W, Al-Dhabi N, Savarimuthu I. Flavonoids: Anticancer Properties; 2017. [Google Scholar]
- 54.Ohno S, Ohno Y, Suzuki N, Soma G-I, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009;29(3):809-815. [PubMed] [Google Scholar]
- 55.Shilpi S, Shivvedi R, Singh A, et al. Vitamin-C: properties, function and application in cancer therapy. J Cancer Prev Curr Res. 2018;9(6):331-334. [Google Scholar]
- 56.Chiu M, Taurino G, Bianchi MG, Kilberg MS, Bussolati O. Asparagine synthetase in cancer: Beyond acute lymphoblastic leukemia. Front Oncol. 2020;9:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batool T, Makky EA, Jalal M, Yusoff MM. A comprehensive review on L-asparaginase and its applications. Appl Biochem Biotechnol. 2016;178(5):900-923. [DOI] [PubMed] [Google Scholar]
- 58.Alrumman S, Mostafa Y, Al-Izran KA, Alfaifi M, Taha T, Elbehairi S. Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci Rep. 2019;9(1):3756-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elhady SS, Eltamany EE, Shaaban AE, et al. Jaceidin flavonoid isolated from Chiliadenus montanus attenuates tumor progression in mice via VEGF inhibition: In Vivo and in silico studies. Plants. 2020;9(8):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alhadrami HA, Hamed AA, Hassan HM, Belbahri L, Rateb ME, Sayed AM. Flavonoids as potential anti-MRSA agents through modulation of PBP2a: A computational and experimental study. Antibiotics. 2020;9(9):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Son Y-O, Kim J, Lim J-C, Chung Y, Chung G-H, Lee J-C. Ripe fruits of Solanum nigrum L. inhibits cell growth and induces apoptosis in MCF-7 cells. Food Chem Toxicol. 2003;41(10):1421-1428. [DOI] [PubMed] [Google Scholar]
- 62.Aboul AM, El FA, Shalaby E, EL H. Potent anticancer and antioxidant activities of active ingredients separated from Solanum nigrum and Cassia italica extracts. J Arid Land Stud. 2014;24(1):145-152. [Google Scholar]
- 63.Li J, Li Q, Feng T, Li K. Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia. 2008;79(7-8):548-556. [DOI] [PubMed] [Google Scholar]