Fig. 8.
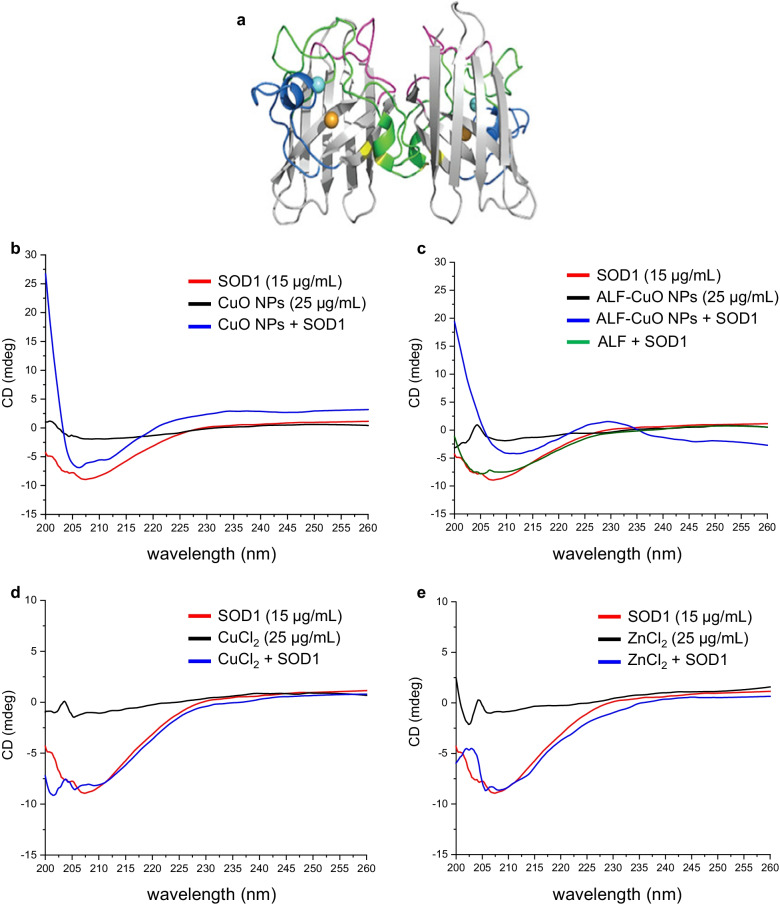
CuO NPs trigger unfolding of SOD1. a SOD1 is a dimeric protein comprised of an eight-stranded β-barrel with one Cu (orange) and Zn (cyan) ion bound in each monomer. Reproduced from: Trist BG, Hilton JB, Hare DJ, Crouch PJ, Double KL. Superoxide dismutase 1 in health and disease: how a frontline antioxidant becomes neurotoxic. Angew Chem Int Ed Engl. 2021;60(17):9215–46 under a Creative Commons license (CC BY 4.0). b–e CD spectroscopy measurements were performed to assess conformational changes in recombinant human SOD1. b CuO NPs decreased the α-helix and β-sheet conformation of the SOD1 protein. c CuO NPs dissolved in ALF (pH 4.5) shifted the α-helix conformation of SOD1 protein from 208 to 212 nm. d CuCl2 caused only a minor effect on the α-helical content, while e ZnCl2 showed no effect. ZnO NPs could not be studied due to assay interference