IMPORTANCE:
Despite high mortality rates of COVID-19-associated pulmonary aspergillosis (CAPA) in the ICU, antifungal prophylaxis remains a subject of debate. We initiated nebulized conventional amphotericin B (c-AmB) as antifungal prophylaxis in COVID-19 patients on invasive mechanical ventilation (IMV).
OBJECTIVES:
To assess the CAPA incidence in COVID-19 patients on IMV treated with and without nebulized c-AmB as antifungal prophylaxis.
DESIGN, SETTING, AND PARTICIPANTS:
Retrospective cohort study of consecutive COVID-19 patients admitted to our adult 17-bed ICU in a university-affiliated general hospital in Ede, The Netherlands, between January 25, 2021, and July 9, 2021. Patients not requiring IMV or transferred from or to another ICU were excluded. From April 9, 2021, daily nebulized amphotericin B in all patients on IMV was initiated.
MAIN OUTCOMES AND MEASURES:
Bronchoscopy with bronchoalveolar lavage (BAL) was performed in case of positive cultures for Aspergillus from the respiratory tract and/or unexplained respiratory deterioration. Incidence of probable and proven CAPA was compared between patients treated with and without nebulized antifungal prophylaxis using Pearson chi-square test.
RESULTS:
A total of 39 intubated COVID-19 patients could be analyzed, of which 16 were treated with antifungal prophylaxis and 23 were not. Twenty-six patients underwent bronchoscopy with BAL. In patients treated with antifungal prophylaxis, the incidence of probable/proven CAPA was significantly lower when compared with no antifungal prophylaxis (27% vs 67%; p = 0.047). Incidence of tracheobronchial lesions and positive Aspergillus cultures and BAL-galactomannan was significantly lower in patients treated with antifungal prophylaxis (9% vs 47%; p = 0.040, 9% vs 53%; p = 0.044, and 20% vs 60%; p = 0.047, respectively). No treatment-related adverse events and no case of proven CAPA were encountered in patients receiving antifungal prophylaxis.
CONCLUSIONS AND RELEVANCE:
Nebulization of c-AmB in critically ill COVID-19 patients on IMV is safe and may be considered as antifungal prophylaxis to prevent CAPA. However, a randomized controlled trial to confirm this is warranted.
Keywords: amphotericin B, antibiotic prophylaxis, Aspergillus, COVID-19, intensive care unit, invasive pulmonary aspergillosis
Direct damage to airway epithelium and immune dysregulation in severe viral pneumonia along with treatment with immunosuppressive agents is known to cause increased susceptibility to fungal superinfections, such as invasive pulmonary aspergillosis (IPA) (1). IPA complicating COVID-19 has been reported in the ICU since the onset of the COVID-19 pandemic (2). Similar to influenza-associated pulmonary aspergillosis (IAPA), COVID-19-associated pulmonary aspergillosis (CAPA) is known to have a nearly two-fold mortality rate compared with the critically ill without pulmonary aspergillosis (3–5). Therefore, prompt diagnostic work-up followed by antifungal therapy is recommended in COVID-19 patients on invasive mechanical ventilation (IMV) with unexplained respiratory deterioration or positive Aspergillus culture from the respiratory tract (1). Since pulmonary lesions on CT imaging are nonspecific in these patients, bronchoscopy and bronchoalveolar lavage (BAL) combined with histopathological examination of lesions is considered the gold standard for diagnosing CAPA (1, 3). However, the early diagnosis of CAPA remains challenging due to difficulty to distinguish between upper respiratory tract Aspergillus colonization and tissue invasive disease (1). Furthermore, the incidence of CAPA may potentially increase by a shift toward ICU admission of COVID-19 patients with underlying (European Organization for Research and Treatment of Cancer/Mycosis Study Group Education and Research Consortium) host factors and broad use of immunotherapy (6). A potentially increasing CAPA incidence together with an associated increased mortality and its diagnostic challenges may justify a prophylactic approach to prevent at risk COVID-19 patients to develop CAPA.
Currently, no antifungal agents are clinically licensed for prophylaxis in the ICU, but three studies have been performed in ICU patients at risk of developing viral pneumonia-associated IPA. One study showed no benefit of posaconazole prophylaxis for preventing IAPA in critically ill patients, which was mainly due to the high proportion of influenza patients that presented with IAPA at the start of ICU admission (7). As the early IAPA cases required immediate antifungal therapy, the study was underpowered to demonstrate a benefit of prophylaxis. However, no influenza patients on antifungal prophylaxis developed IAPA during ICU admission. Another study was performed in 132 COVID-19 patients in the ICU, in which only one patient received interleukin-6 blockade as immunosuppressive treatment besides corticosteroids. The CAPA incidence was 1.4% in the 75 patients receiving prophylaxis by posaconazole compared with 17.5% in those not receiving prophylaxis (8). Furthermore, Van Ackerbroeck et al (9) investigated an alternative prevention strategy by nebulizing liposomal amphotericin B (L-AmB) twice weekly in COVID-19 patients on IMV in the ICU and observed a reduced incidence of CAPA in patients receiving antifungal prophylaxis. Aerosolized AmB achieves drug levels in lung tissue and has been shown to result in a substantial decrease in IPA in hematologic-oncologic patients and to be cost saving compared with no prophylaxis (10–12). Furthermore, administration of antifungals through inhalation avoids drawbacks associated with systemic prophylaxis including (nephro)toxicity, selection of (azole) resistance, and drug-drug interactions, which is a recognized problem of mold-active azoles (10, 13). An important advantage of nebulized drug administration is a potential topical effect in patients presenting with invasive Aspergillus tracheobronchitis, which is a common manifestation in virus-associated IPA and carries a very high mortality rate (14, 15). However, evidence supporting the use of nebulized AmB is limited, although several studies indicate that this administration route is not associated with local side effects in the lung (10, 11).
Since introducing tocilizumab alongside dexamethasone as standard therapeutic regimen for severe COVID-19 in our ICU, we have encountered a high number of COVID-19 patients developing CAPA. Therefore, we initiated nebulized conventional amphotericin B (c-AmB) deoxycholate as antifungal prophylaxis in COVID-19 patients on IMV. The objective of this study was to assess the CAPA incidence among COVID-19 patients on IMV treated with and without nebulized c-AmB.
METHODS
Study Design, Setting, and Patient Selection
We conducted a single-center retrospective cohort study among patients with real-time polymerase chain reaction (RT-PCR)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection admitted to our 17-bed mixed medical-surgical ICU in a University-affiliated general hospital (Gelderse Vallei Hospital, Ede, The Netherlands). The study cohort was recruited from January 25, 2021, to July 9, 2021. At that time, tocilizumab alongside dexamethasone became the standard of care in our ICU. The study period represents the fourth COVID-19 wave in the Netherlands. All consecutively admitted patients, greater than or equal to 18 years old, with a PCR confirmed SARS-CoV-2 infection and requiring ICU admission for respiratory support were included in the cohort. Patients transferred to another ICU before extubation or transferred from another ICU more than 24 hours after initiation of IMV were excluded. This study was performed following the ethical standards of the Institutional Research Committee and in accordance with the Declaration of Helsinki. The study was approved by our Institutional Review Board (approval nr. 2101-009).
Patient Management and Nebulization of Amphotericin B
From January 25, 2021, a single dose of tocilizumab (8 mg/kg, maximum of 800 mg) alongside dexamethasone (6 mg once daily) for 10 days and ceftriaxone (2,000 mg once daily) for 4 days was standard of care in COVID-19 patients in our ICU. All patients on IMV were treated with selective oral decontamination comprising nystatin, colistin, and tobramycin topically administered four times daily. Sputum was cultured for bacteria and fungi at ICU admission and tracheal aspirate (TA) was cultured twice weekly following endotracheal intubation. Positive cultures with Aspergillus species were analyzed using quantitative RT-PCR assay with detection of azole resistance as described previously (16, 17). Antifungal prophylaxis was implemented on April 9, 2021, with all COVID-19 patients who were on IMV receiving daily 20 mg nebulized c-AmB. Ten mg bid was given between April 9 and April 30. After April 30, we switched to four times daily 5 mg. Nebulization was performed placing Intersurgical Cirrus 2 nebulizer (Intersurgical Benelux, Uden, The Netherlands) at the end of the inspiratory breathing circuit of the Hamilton-C3 or Hamilton-S1 ventilator for 30 minutes using humidified air. An additional medication filter was placed at the end of the expiratory breathing circuit to prevent clogging of the expiratory valve and was replaced after every nebulization. Ventilator problems directly following nebulization of c-AmB, such as clogging of the expiratory filters or bronchospasms, were considered treatment-related adverse effects.
CAPA Classification
Bronchoscopy for inspection of trachea and bronchi, followed by BAL, was performed in case of positive cultures for Aspergillus species from the respiratory tract and/or unexplained respiratory deterioration. Samples were processed for galactomannan (Platelia Aspergillus; Bio-Rad Laboratories, Marnes la Coquette, France), Blankophor P staining, and culture. Negative BAL-galactomannan, culture-negative samples, or a BAL sample of too high viscosity to reliably assess galactomannan were analyzed using a quantitative RT-PCR assay for Aspergillus DNA. In case tracheobronchial lesions were noticed at bronchoscopy, a biopsy of the lesion was taken for histopathological examination if possible. If fungal hyphae were not detected or invasive growth was absent at histopathological examination, specimens were analyzed using Aspergillus PCR. Additionally, serum-galactomannan was determined at the discretion of the attending intensivist. A galactomannan optical density (OD) index greater than or equal to 1.0 in BAL and greater than or equal to 0.5 in serum were considered positive for CAPA. According to the 2020 European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) consensus criteria, CAPA was classified into unlikely, probable or proven following BAL and histopathological results (1). No cases could be classified as possible CAPA as a nonbronchoscopic BAL was not part of standard of care in our center. Patients with high suspicion of CAPA, for example, with positive Aspergillus markers in BAL and/or with evidence for invasive tracheobronchitis during bronchoscopy, were treated with voriconazole and micafungin, in accordance with the national guideline.
Data Collection
Variables were assessed at hospital admission and included age, sex, and body mass index. Concerning COVID-19 disease, standard laboratory findings at ICU admission, days since onset of COVID-19 disease at ICU admission, and IMV and ICU admission duration were recorded. Patient information, outcome, and microbiological records were collected from local electronic medical record systems Metavision (iMDsoft, Tel Aviv, Israel) and NeoZIS (MI Consultancy, Katwijk, The Netherlands) until 90 days after ICU admission. Data were collected and handled anonymously.
Statistical Methods
A retrospective analysis of prospectively recorded data was performed. For descriptive analyses, categorical variables are presented as counts alongside percentages. Continuous variables are presented as means and sd if normally distributed or as medians and interquartile ranges (IQRs) if not normally distributed. Normality was tested using Shapiro-Wilk and Kolmogorov-Smirnov tests and visual inspecting of plots. For comparison between groups, Student t tests were used in case continuous data were normally distributed; otherwise, Mann-Whitney U tests were used. Categorical variables were compared using Pearson chi-square test. All statistical analyses were performed with IBM SPSS statistics Version 27 (IBM Corp, Armonk, NY). p values of less than 0.05 were considered statistically significant.
RESULTS
General Characteristics
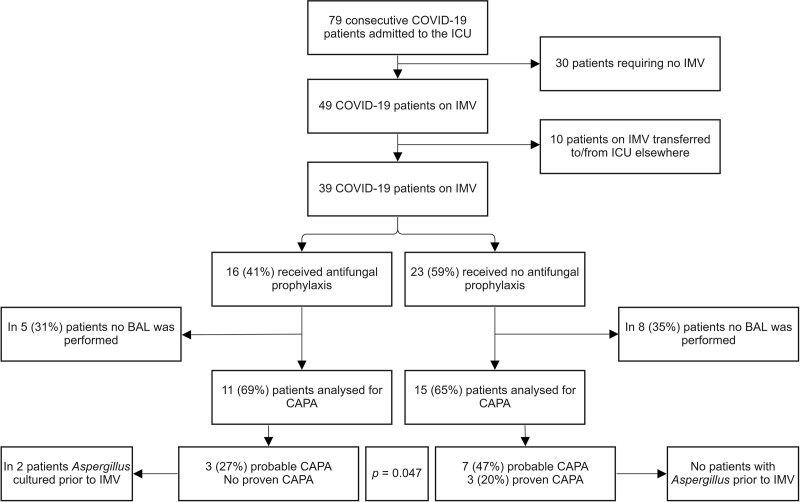
During the study period, 79 patients diagnosed with COVID-19 were admitted to our ICU. Of these, 49 (62%) required endotracheal intubation for IMV. The remaining patients were supported by noninvasive ventilation or high-flow nasal oxygen therapy. Ten patients could not be included because of transfer from another ICU or early transfer to another ICU due to lack of ICU capacity. Eventually, 39 patients on IMV were analyzed to study the effect of antifungal prophylaxis by nebulization. Of these, 16 (41%) received antifungal prophylaxis and 23 (59%) did not. Cohort distribution is illustrated in Figure 1. Nine patients (56%) treated with antifungal prophylaxis and 15 patients (65%) not treated with antifungal prophylaxis were on IMV within 24 hours of ICU admission. Median time until start IMV was 2 days (IQR, 1–6 d) and 1 day (IQR, 1–2 d) in patients treated with and without antifungal prophylaxis, respectively. All patients were treated with dexamethasone. One patient did not receive a dose of tocilizumab because of severe hepatic dysfunction. Comparison of patients who received prophylactic antifungal treatment following endotracheal intubation and those who did not, regarding demographics, ICU admission, laboratory, and microbiological characteristics, are presented in Table 1.
Figure 1.
Flowchart of admitted COVID-19 patients, including main findings regarding COVID-19-associated pulmonary aspergillosis (CAPA) incidence in patients who underwent a bronchoalveolar lavage (BAL) procedure. IMV = invasive mechanical ventilation.
TABLE 1.
The Demographic, ICU, Microbiological, and Histopathological Characteristics of COVID-19 Patients Treated With and Without Antifungal Prophylaxis
| Patient Characteristics | All (n = 39) | BAL (n = 26) | p | |||
|---|---|---|---|---|---|---|
| Antifungal Prophylaxis (n = 16) | No Antifungal Prophylaxis (n = 23) | p | Antifungal Prophylaxis (n = 11) | No Antifungal Prophylaxis (n = 15) | ||
| Demographics | ||||||
| Age, yr | 65 (7) | 65 (8) | 0.892 | 65 (7) | 68 (8) | 0.351 |
| Body mass index, kg/m2 | 29.9 (5.7) | 20.2 (5.7) | 0.870 | 28.9 (5.0) | 29.3 (4.4) | 0.850 |
| Male, n (%) | 11 (69) | 14 (61) | 0.614 | 9 (82) | 10 (67) | 0.390 |
| Main comorbidities, n (%) | ||||||
| Acute Physiology and Chronic Health Evaluation IV | 62 (16) | 68 (20) | 0.349 | 61 (17) | 74 (18) | 0.060 |
| Cardiac disease | 4 (25) | 8 (35) | 0.515 | 3 (27) | 7 (48) | 0.315 |
| Diabetes mellitus type II | 3 (19) | 8 (35) | 0.274 | 2 (19) | 6 (40) | 0.234 |
| Chronic respiratory disease | 8 (50) | 8 (35) | 0.342 | 5 (46) | 5 (33) | 0.530 |
| History of smoking | 2 (13) | 8 (35) | 0.117 | 2 (18) | 4 (27) | 0.612 |
| Renal insufficiency | 1 (13) | 3 (6) | 0.492 | 2 | 0 | 0.207 |
| Active oncologic disease | 1 (6) | 1 (4) | 0.791 | 1 (9) | 1 (7) | 0.819 |
| European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium host factor | 0 | 1 (4) | 0.398 | 0 | 1 (7) | 0.382 |
| ICU characteristics | ||||||
| C-reactive protein at admission, mg/L | 109 (62–163) | 103 (74–139) | 0.771 | 109 (73–146) | 109 (63–164) | 0.827 |
| Procalcitonin at admission, μg/L | 0.32 (0.27–0.80) | 0.31 (0.12–0.36) | 0.736 | 0.28 (0.21–0.82) | 0.33 (0.21–0.68) | 0.884 |
| Acute kidney injury at admission, n (%) | 2 (13) | 6 (26) | 0.270 | 1 (9) | 5 (33) | 0.147 |
| COVID-19 disease day at admission | 7 (6–9) | 9 (7–11) | 0.320 | 8 (7–11) | 8 (7–11) | 0.683 |
| Treatment with tocilizumab, n (%) | 16 (100) | 22 (96) | 0.398 | 11 (100) | 14 (93) | 0.382 |
| Days until first (first) bronchoscopy | 9 (3) | 8 (2) | 0.387 | 8.0 (3) | 8 (2) | 0.387 |
| Length of IMV, d | 17 (15–34) | 11 (7–17) | 0.116 | 16 (15–31) | 17 (11–23) | 0.567 |
| ICU length of stay, d | 26 (17–36) | 17 (9–28) | 0.039 | 31 (21–40) | 22 (16–33) | 0.073 |
| Hospital length of stay, d | 33 (23–43) | 23 (16–43) | 0.270 | 35 (24–48) | 35 (21–45) | 0.683 |
| 90-d mortality, n (%)a | 3 (19) | 5 (22) | 0.820 | 3 (27) | 5 (33) | 0.741 |
| Microbiological characteristics, n (%) | ||||||
| Aspergillus cultured from sputum/TA | 5 (31) | 12 (52) | 0.195 | 5 (56) | 10 (91) | 0.069 |
| Aspergillus cultured from TA after start IMV | 3 (60) | 11 (93) | 0.119 | 3 (60) | 10 (100) | 0.040 |
| Days of IMV until first Aspergillus cultured | 7 (1–8) | 5 (3–6) | 0.909 | 7 (1–8) | 5 (4–6) | 0.788 |
| BAL Aspergillus culture positive | — | — | — | 1 (9) | 8 (53) | 0.044 |
| BAL-galactomannan optical density > 1.0 | — | — | — | 2 (20) | 9 (60) | 0.048 |
| Tracheobronchial lesion(s) at bronchoscopy | — | — | — | 1 (9) | 7 (47) | 0.040 |
| Bronchoscopic biopsy | — | — | — | 1 (9) | 4 (27) | 0.154 |
| Biopsy showing fungi | — | — | — | 0 | 4 (100) | — |
| COVID-19-associated pulmonary aspergillosis | ||||||
| Probable | 3 (19) | 7 (30) | 0.279 | 3 (27) | 7 (47) | 0.315 |
| Proven | 0 | 3 (13) | 0.106 | 0 | 3 (20) | 0.115 |
| Probable or proven | 3 (19) | 10 (43) | 0.107 | 3 (27) | 10 (67) | 0.047 |
BAL = bronchoalveolar lavage, IMV = invasive mechanical ventilation, TA = tracheal aspirate.
aICU, hospital, and 90-d mortality was equal in the whole cohort.
Continuous variables are presented as mean (sd) and compared using a Student t test or as median (interquartile range) and compared using a Mann-Whitney U test. Dichotomous variables are presented as number (percentage) and compared using a Pearson χ2 test.
According to the 2020 European Confederation of Medical Mycology/International Society for Human and Animal Mycology consensus criteria, COVID-19-associated pulmonary aspergillosis (CAPA) was classified into unlikely, probable or proven following BAL and histopathological results (1). Nonbronchoscopic bronchoalveolar lavages were not part our centers standard of care, and therefore, no cases could be classified as possible CAPA.
Moment of Performing BAL
In total, 26 patients (67%) underwent a bronchoscopy. Of these, in 12 patients (46%), a BAL was performed due to respiratory deterioration, in 10 patients (38%) due to positive sputum/TA cultures with Aspergillus species in patients without respiratory improvement, and in four patients (15%), both occurred. The mean time to the first BAL was 8 days (± 2 d) from ICU admission and 17 days (± 4 d) from the onset of COVID-19 symptoms. BAL was repeated due to further respiratory deterioration in three patients and unexplained fever and/or rising infection parameters in four at a mean time of 19 days (± 7 d) from ICU admission. Reiteration of BAL diagnostics revealed no new information concerning CAPA.
CAPA Characteristics
Main findings regarding CAPA incidence are depicted in Figure 1. Probable or proven CAPA was detected in 13 patients (50%) who underwent BAL. The mean time at which first Aspergillus was cultured from sputum/TA was 6 days (± 3 d) after ICU admission. In all culture-positive patients, Aspergillus fumigatus was cultured, which were in vitro susceptible to AmB and mold-active azoles, except for one isolate that was azole resistant.
Aspergillus was cultured from sputum in three patients before or at the initiation of IMV. BAL-galactomannan was performed in 25 patients and was positive on the first BAL-fluid in 11 patients (44%) with a mean OD index value of 4.7 (± 2.0). In one patient, BAL-galactomannan could not reliably be performed due to high viscosity of the sample, in which Aspergillus PCR was negative. Serum-galactomannan was obtained in 12 patients (26%) and tested negative in all with a median index value of 0.06 (IQR, 0.04–0.15). Tracheobronchial lesions were observed in eight patients (31%), of which five underwent mucosal biopsy for histopathological analysis. Of these, four specimens (80%) revealed fungal hyphae, of which two showed evidence of invasive growth. Aspergillus DNA was detected by PCR in one of the two specimens without microscopic evidence for fungal hyphae. In the other patient, not enough bronchoscopic retrieved tissue was available for PCR, and therefore, lung tissue obtained during autopsy was analyzed for Aspergillus DNA by RT-PCR and tested negative. The biopsy that revealed no fungal hyphae by microscopy tested negative for Aspergillus DNA by RT-PCR.
The mean time until the start of systemic antifungal therapy in the patients with CAPA was 9 days (± 3 d) from ICU admission, 8 days (± 3 d) from initiation of IMV, and 19 days (± 5 d) from the onset of COVID-19 diagnosis. Comparison of patients with probable/proven CAPA and patients unlikely to have CAPA, regarding patient characteristics, main comorbidities, and ICU characteristics, are presented in Table 2 for the patients that underwent a BAL. ICU and 90-day mortality of patients who underwent BAL and were diagnosed with probable/proven CAPA was 63% versus 38% in CAPA unlikely (p = 0.395). In probable/proven CAPA, the median galactomannan index value on the first BAL-fluid was 4.74 (IQR, 1.31–5.95). Individual ICU and microbiological characteristics of patients with probable/proven CAPA are presented in the Supplemental Table (http://links.lww.com/CCX/A992).
TABLE 2.
The Demographic and ICU Characteristics of COVID-19 Patients With and Without Probable/Proven COVID-19-Associated Pulmonary Aspergillosis
| Patient Characteristics | CAPA Unlikely (n = 13) | Probable or Proven CAPA (n = 13) | p |
|---|---|---|---|
| Demographics | |||
| Age, yr | 65 (8) | 68 (7) | 0.248 |
| Body mass index, kg/m2 | 29.1 (4.8) | 29.2 (4.6) | 0.967 |
| Male, n (%) | 10 (77) | 9 (69) | 0.658 |
| Main comorbidities | |||
| Acute Physiology and Chronic Health Evaluation IV | 62 (16) | 75 (18) | 0.054 |
| Cardiac disease, n (%) | 2 (15) | 8 (62) | 0.016 |
| Diabetes mellitus type II, n (%) | 0 | 8 (62) | 0.001 |
| Chronic respiratory disease, n (%) | 6 (46) | 4 (31) | 0.420 |
| History of smoking, n (%) | 1 (8) | 5 (39) | 0.063 |
| Renal insufficiency, n (%) | 0 | 2 (15) | 0.141 |
| Active oncologic disease, n (%) | 0 | 2 (15) | 0.141 |
| European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium host factor | 0 | 1 (8) | 0.308 |
| ICU characteristics | |||
| COVID-19 disease day at admission | 8 (2) | 10 (3) | 0.319 |
| Days until (first) bronchoscopy | 8 (2) | 8 (2) | 0.521 |
| Length of invasive mechanical ventilation, d | 16 (13–24) | 17 (12–29) | 0.644 |
| Antifungal prophylaxis, n (%) | 8 (62) | 3 (23) | 0.047 |
| ICU length of stay, d | 23 (19–35) | 29 (17–41) | 0.723 |
| Hospital length of stay, d | 34 (24–43) | 40 (23–58) | 0.367 |
| 90-d mortality, n (%) | 3 (23) | 5 (38) | 0.395 |
CAPA = COVID-19-associated pulmonary aspergillosis.
Continuous variables are presented as mean (sd) and compared using a Student t test or as median (interquartile range) and compared using a Mann-Whitney U test. Dichotomous variables are presented as numbers (percentage) and compared using a χ2 test. According to the 2020 European Confederation of Medical Mycology/International Society for Human and Animal Mycology consensus criteria, CAPA was classified into unlikely, probable or proven following bronchoalveolar lavage (BAL) and histopathological results (1). A nonbronchoscopic BAL was not part of our standard of care, and therefore, no cases could be classified as possible CAPA.
Antifungal Prophylaxis
Incidence of tracheobronchial lesions, positive Aspergillus cultures and positive BAL-galactomannan was significantly lower in patients receiving nebulized c-AmB compared with controls (9% vs 47%; p = 0.040, 9% vs 53%; p = 0.044, and 20% vs 60%; p = 0.047, respectively). Of the patients who underwent BAL, three patients (27%) on antifungal prophylaxis and 10 patients (67%) not receiving antifungal prophylaxis developed probable/proven CAPA (p = 0.047). There was not one case of proven CAPA observed in patients receiving antifungal prophylaxis. Two of the three probable CAPA cases in patients receiving nebulized c-AmB had positive sputum cultures prior to IMV, while in none of the probable/proven CAPA cases not receiving antifungal prophylaxis Aspergillus was cultured prior to IMV. Mean time to diagnosis of CAPA was 10 days (± 3 d) in patients receiving c-AmB and 9 days (± 3 d) in patients receiving no antifungal prophylaxis. Nebulization of c-AmB 10 mg bid led to clogging of the expiratory filters during administration in two patients without any serious adverse events. After switching to 5 mg four times daily, no filter problems or other treatment-related adverse events occurred. Ninety-day mortality was 21% within the whole cohort. Three patients in the group with c-AmB died (one of cryptogenic organizing pneumonia, one of ventilator-associated pneumonia, and one of central pulmonary embolism complicated with infected lung infarction) and five patients in the group without c-AmB died (one with probable and one of proven CAPA, two of cryptogenic organizing pneumonia, and one of disseminated mucormycosis infection). Four out of 10 probable/proven CAPA patients without antifungal prophylaxis versus one out of three probable/proven CAPA patients treated with prophylaxis deceased within 90 days of ICU admission.
DISCUSSION
In this retrospective cohort study, we observed a lower incidence of probable/proven CAPA in patients on IMV receiving nebulized c-AmB compared with patients not receiving antifungal prophylaxis. The CAPA incidence by bronchoscopy with BAL was 67% in the cohort not receiving prophylaxis compared with 27% in those on prophylaxis. In addition to three cases of proven IPA compared with no proven IPA in the patients without antifungal prophylaxis, nebulized c-AmB was also associated with a lower number of positive Aspergillus cultures from the upper respiratory tract, indicating that prophylaxis may reduce Aspergillus colonization. It has been suggested that respiratory tract colonization precedes the development of tissue invasion and subsequent angioinvasion (15), thus suppression or eradication of Aspergillus from the respiratory tract may be an important factor in preventing CAPA. Results of Gangneux et al (3) may indicate an association between any positive Aspergillus culture and increased mortality, even if they could not be classified as CAPA, which suggests that “decolonization” of the respiratory tract may help to improve patient outcome. Aside from clogging of the respiratory expiration filter when administrating a high concentration of 10 mg c-AmB two times daily, we observed no treatment-related adverse effects after halving the concentration and administering this as 5 mg four times daily. Our results suggest that antifungal prophylaxis with nebulized c-AmB is feasible and safe in COVID-19 patients on IMV and that these patients may benefit from it in order to prevent CAPA, especially in ICUs encountering high incidence rates of CAPA.
The frequency of probable/proven CAPA in our nonprophylaxis COVID-19 cohort was very high, 67% in IMV patients who had undergone BAL and 43% when patients without bronchoscopy and BAL are included. Previous European studies using the ECMM/ISHAM 2020 case definition reported CAPA frequencies between 10% and 20% (3, 5, 8, 18–20), but the proportion of ventilated patients and patients receiving interleukin-6 inhibitors and/or dexamethasone in these studies was lower compared with our cohort. In addition, fear of contamination by performing a BAL has diminished during the subsequent COVID waves increasing the detection rate. Furthermore, twice weekly monitoring of respiratory samples with a low threshold to perform bronchoscopy may have contributed to the high number of cases we encountered.
Our study results are in agreement with the study of Van Ackerbroeck et al (9), who evaluated twice weekly nebulized L-AmB in a cohort of 50 critically ill COVID-19 patients. Similar to our findings, both frequency of CAPA and Aspergillus colonization were significantly lower in patients receiving nebulized antifungal prophylaxis. However, compared with the cohort of Van Ackerbroeck et al (9), the proportion of patients receiving interleukin-6 inhibitors was higher in our study (16% compared with 97%, respectively), which may correspond with a higher risk to develop secondary fungal infections (3, 17). As interleukin-6 inhibitors and corticosteroids remain the cornerstone for treating critically ill COVID-19 patients (21, 22), the results of the present cohort study may be of value to other medical ICUs encountering high incidence rates of CAPA.
Invasive Aspergillus tracheobronchitis is a highly lethal manifestation of IPA, which was shown to be associated with a mortality rate of 90% in patients with IAPA admitted to the ICU (14). The frequency of Aspergillus tracheobronchitis in CAPA is less well defined, but in our cohort, eight of 26 patients (31%) showed evidence for endotracheal lesions, of which four showed evidence for fungal hyphae in patients without antifungal prophylaxis. Although the mortality rate associated with invasive Aspergillus tracheobronchitis in CAPA remains unclear, systemic antifungal therapy alone might be suboptimal to treat this condition as intraluminal fungal growth may not be inhibited. Nebulized antifungals have been recommended as addition to systemic antifungals in the treatment of invasive tracheobronchitis in lung transplant recipients (23), but evidence supporting its use is lacking. In our study, we observed a significant lower frequency of tracheobronchitis in patients receiving nebulized c-AmB compared with standard of care (9% vs 47%) indicating that topical endotracheal AmB exposure may help to prevent the development of tracheobronchitis. In virus-associated IPA, this may be an important advantage over systemic antifungal prophylaxis. One single center showed that systemic azole prophylaxis, mainly posaconazole, was highly effective in reducing CAPA in critically ill COVID-19 patients (8). Among 75 patients receiving azole prophylaxis only one patient developed CAPA compared with eight of 57 patients without prophylaxis (8). However, the number of invasive Aspergillus tracheobronchitis cases was not reported nor the effect of systemic prophylaxis on Aspergillus colonization.
In our cohort, three out of 11 patients in whom a bronchoscopy was performed a probable CAPA was detected, despite the nebulization of c-AmB. One of these patients was diagnosed with CAPA based solely on a positive BAL-galactomannan, while BAL PCR and cultures remained negative for Aspergillus, which may indicate a false positive BAL-galactomannan (24, 25). The other two patients had positive sputum cultures prior to intubation, indicating Aspergillus colonization or infection before prophylaxis was initiated. This indicates that prophylaxis may need to commence earlier during ICU admission, which might involve nebulizing AmB in COVID-19 patients on high-flow nasal cannula oxygen support or noninvasive ventilation in high-risk patients. Administration of nebulized L-AmB in hematology patients for the prevention of IPA underscores the feasibility of this approach in nonendotracheal intubated patients (10, 11).
Our study has several limitations including the small, single-center derived cohort, possibly limiting its generalizability. Also, screening for CAPA by bronchoscopy was at the discretion of the attending physician, potentially introducing selection bias. However, we observed similar baseline characteristics in those treated with and without antifungal prophylaxis who underwent bronchoscopy.
Including the current study, to date, three single-center studies have shown a beneficial effect of antifungal prophylaxis in critically ill COVID-19 patients (8, 9), alongside one ICU reporting to have initiated antifungal prophylaxis with inhaled L-AmB after encountering high CAPA incidence rates (26). Although the studies show a lower frequency of CAPA and Aspergillus colonization in patients receiving prophylaxis, a survival benefit was not demonstrated possibly due to the limited sample sizes. We observed a longer duration of ICU stay in patients treated with c-AmB. However, it is impossible to draw firm conclusions on such multifactorial determined outcome measures in a small sample cohort. It would require a large randomized controlled trial to investigate the implications of antifungal prophylaxis for patient outcome. However, both systemic and nebulized prophylactic strategies come with certain challenges. Systemic azole prophylaxis may involve posaconazole or isavuconazole, but there are limited data on the pharmacology of these agents in critically ill patients, drug-drug interactions may be frequent and need to be managed, and there is concern regarding selection of azole resistance (13, 27). For nebulized antifungals, little is known regarding drug deposition in the lung and airways in ventilated individuals, optimal particle size, dose, and dosing frequency. Although most studies have used a formulation of AmB, new azole drugs, such as opelconazole, are in clinical development for inhalation, which may also be considered for this indication (28). The benefit of antifungal prophylaxis will further depend on the frequency of CAPA and characteristics of critically ill COVID-19 cases, which depends on evolution of the COVID, vaccination coverages, and efficacy of SARS-CoV-2 treatments.
CONCLUSIONS
High mortality rates and increasing incidence of CAPA in the currently severely immunocompromised COVID-19 patients underscores the need for an antifungal prophylaxis strategy. Daily nebulization of c-AmB was safe in COVID-19 patients on IMV and may be effective to reduce the incidence of CAPA. However, a confirmative randomized controlled trial is warranted, focusing on its effectiveness in preventing IPA and improving patient outcome.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
This study was conducted at Gelderse Vallei Hospital, Ede, The Netherlands.
REFERENCES
- 1.Koehler P, Bassetti M, Chakrabarti A, et al. ; European Confederation of Medical Mycology; International Society for Human Animal Mycology; Asia Fungal Working Group; INFOCUS LATAM/ISHAM Working Group; ISHAM Pan Africa Mycology Working Group; European Society for Clinical Microbiology; Infectious Diseases Fungal Infection Study Group; ESCMID Study Group for Infections in Critically Ill Patients; Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy; Medical Mycology Society of Nigeria; Medical Mycology Society of China Medicine Education Association; Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology; Association of Medical Microbiology; Infectious Disease Canada: Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21:e149–e16233333012 [Google Scholar]
- 2.van Arkel ALE, Rijpstra TA, Belderbos HNA, et al. : COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 2020; 202:132–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangneux JP, Dannaoui E, Fekkar A, et al. : Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir Med 2021; 10:180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Yang X, Lv Z, et al. : Risk factors for invasive aspergillosis in patients admitted to the intensive care unit with coronavirus disease 2019: A multicenter retrospective study. Front Med (Lausanne) 2021; 8:753659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feys S, Almyroudi MP, Braspenning R, et al. : A visual and comprehensive review on COVID-19-associated pulmonary aspergillosis (CAPA). J Fungi (Basel) 2021; 7:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verweij PE, van de Veerdonk FL: Managing secondary fungal infections in severe COVID-19: How to move forward? Lancet Respir Med 2021; 10:127–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderbeke L, Janssen NAF, Bergmans DCJJ, et al. ; Dutch-Belgian Mycosis Study Group: Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): A randomised, open-label, proof-of-concept trial. Intensive Care Med 2021; 47:674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzl S, Reisinger AC, Posch F, et al. : Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: An observational study. Crit Care 2021; 25:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Ackerbroeck S, Rutsaert L, Roelant E, et al. : Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care 2021; 25:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rijnders BJ, Cornelissen JJ, Slobbe L, et al. : Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: A randomized, placebo-controlled trial. Clin Infect Dis 2008; 46:1401–1408 [DOI] [PubMed] [Google Scholar]
- 11.Duckwall MJ, Gales MA, Gales BJ: Inhaled amphotericin B as aspergillosis prophylaxis in hematologic disease: An update. Microbiol Insights 2019; 12:1178636119869937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong GL, Broekman F, Polinder S, et al. : Aerosolised liposomal amphotericin B to prevent aspergillosis in acute myeloid leukaemia: Efficacy and cost effectiveness in real-life. Int J Antimicrob Agents 2015; 46:82–87 [DOI] [PubMed] [Google Scholar]
- 13.Verweij PE, Ananda-Rajah M, Andes D, et al. : International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 2015; 21–22:30–40 [DOI] [PubMed] [Google Scholar]
- 14.Nyga R, Maizel J, Nseir S, et al. : Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza. A clinical trial. Am J Respir Crit Care Med 2020; 202:708–716 [DOI] [PubMed] [Google Scholar]
- 15.van de Veerdonk FL, Brüggemann RJM, Vos S, et al. : COVID-19-associated Aspergillus tracheobronchitis: The interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med 2021; 9:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi E, Hedayati MT, Zoll J, et al. : Discrimination of aspergillosis, mucormycosis, fusariosis, and scedosporiosis in formalin-fixed paraffin-embedded tissue specimens by use of multiple real-time quantitative PCR assays. J Clin Microbiol 2016; 54:2798–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Linden JW, Snelders E, Arends JP, et al. : Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. J Clin Microbiol 2010; 48:1478–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen NAF, Nyga R, Vanderbeke L, et al. : Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis1. Emerg Infect Dis 2021; 27:2892–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prattes J, Wauters J, Giacobbe DR, et al. ; ECMM-CAPA Study Group: Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect 2021; 28:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ergün M, Brüggemann RJM, Alanio A, et al. : Aspergillus test profiles and mortality in critically ill COVID-19 patients. J Clin Microbiol 2021; 59:e0122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators: Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group: Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson TF, Thompson GR, III, Denning DW, et al. : Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:e1–e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lheureux O, Montesinos I, Taton O, et al. : False-positive galactomannan assay in broncho-alveolar lavage after enteral nutrition solution inhalation: A case report. JMM Case Rep 2017; 4:e005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellière S, Dudoignon E, Voicu S, et al. : Combination of mycological criteria: A better surrogate to identify COVID-19 associated pulmonary aspergillosis patients and evaluate prognosis? J Clin Microbiol 2022; 60:JCM0216921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano MC, Narváez-Chávez G, López-Olivencia M, et al. : Inhaled amphotericin B lipid complex for prophylaxis against COVID-19-associated invasive pulmonary aspergillosis. Intensive Care Med 2021; 48:360–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seyedmousavi S, Mouton JW, Verweij PE, et al. : Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev Anti Infect Ther 2013; 11:931–941 [DOI] [PubMed] [Google Scholar]
- 28.Hoenigl M, Sprute R, Egger M, et al. : The antifungal pipeline: Fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021; 81:1703–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]