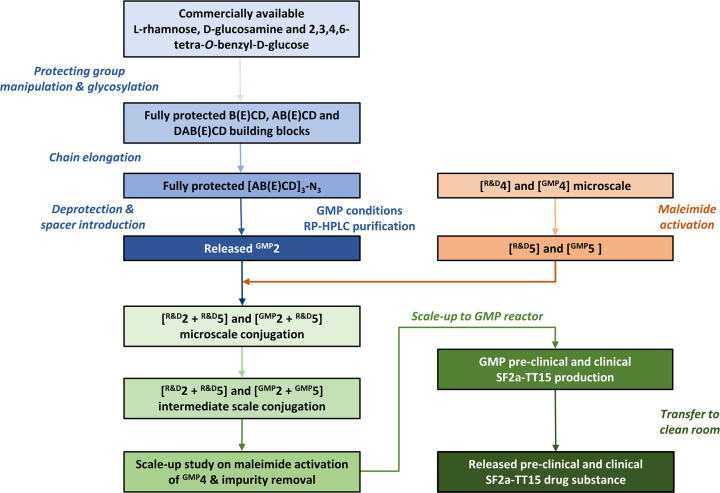
Figure 2.
Overview of process development and SF2a-TT15 bulk release. Blue panels: synthesis of GMP-grade precursor 2 (GMP2).1 Orange panels: maleimide activation of 4 and conjugation reaction with the introduction of 2. Green panels: process development for GMP manufacturing of SF2a-TT15 from precursor 2. Release of the (pre)-clinical conjugate vaccine DS was based on impurity assessment (NMR), free and total carbohydrate content (HPAEC-PAD), osmolality, pH, endotoxin, and aggregate content (HPSEC). HPAEC-PAD: high-performance anion-exchange chromatography with pulsed amperometric detection, HPSEC: high-performance size exclusion chromatography.