Abstract
The exposure of healthcare workers (HCWs) to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has been a major concern since the beginning of the coronavirus disease 2019 (COVID‐19) pandemic. The study aimed to investigate the relationship between vaccination status and the status of catching COVID‐19 in HCWs working in a Training and Research Hospital in Turkey, and the clinical course of the disease in those who were caught. The vaccination status of 1279 HCWs working at Siirt Training and Research Hospital during the period when the SARS‐CoV‐2 Delta variant was dominant, their cases of catching COVID‐19 during this period, and the clinical course of the disease in patients with COVID‐19 were investigated retrospectively. We found that the rate of COVID‐19 transmission was lowest in fully vaccinated HCWs (p < 0.05). The rate of COVID‐19 transmission in HCWs who received two doses of BioNTech vaccine (4.4%) and two doses of CoronaVac+ one dose of BioNTech vaccines (2.7%) was considerably lower than those without vaccination (26.2%) (p < 0.05). The transmission rate was lowest among those vaccinated with two doses of CoronaVac+ one dose of BioNTech. Hospitalization was not required in fully vaccinated HCWs. The lymphocyte count was found to be significantly higher in fully vaccinated patients than incompletely vaccinated and unvaccinated patients. Although C‐reactive protein (CRP), d‐dimer, and ferritin values were higher in unvaccinated and partially vaccinated patients than in fully vaccinated patients, the differences were not statistically significant. As a result, the transmission rate of COVID‐19 was lowest in fully vaccinated HCWs and in those vaccinated with two doses of CoronaVac+ one dose of BioNTech. In fully vaccinated HCWs, hospitalization was not needed.
Keywords: BioNTech, CoronaVac, COVID‐19, healthcare workers
1. INTRODUCTION
Over 545 million people have died worldwide since World Health Organization (WHO) declared coronavirus disease 2019 (COVID‐19) a pandemic on March 11, 2020. 1 In addition, over 88 000 people have died in Turkey so far. 2 Private and public institutions have been unprecedented international efforts to develop a vaccine against the disease‐causing severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). 3 The exposure of healthcare workers (HCWs) to SARS‐CoV‐2 has been a major concern since the beginning of the COVID‐19 pandemic. 4 COVID‐19 vaccinations, combined with personal protective measures, have been deemed the most effective way to stop the virus from spreading. 5
While to date, nonpharmaceutical interventions for instance social distancing, face masks, and contact tracing have been the mainstay of health policy strategies to decrease the viral spread and limit requests for healthcare, new COVID‐19 vaccines are starting to change that. 6 For existing vaccine‐effectiveness estimates, the focus is on the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer‐BioNTech), the ChAdOx1 nCoV‐19 vaccine (Oxford‐AstraZeneca), and the mRNA‐1273 vaccine (Moderna). 7 , 8 , 9 , 10 In addition, the inactivated SARS‐CoV‐2 vaccine (CoronaVac) has been approved for emergency use in 22 countries, mostly low‐ and middle‐income countries. Despite its global importance, evidence of the vaccine's efficacy or effectiveness is limited. 6 Siirt Training and Research Hospital has been serving as a pandemic hospital since the beginning of the pandemic. For this reason, healthcare professionals come into contact with a large number of COVID‐19 patients every day. Since the continuity of the service depends on the health status of the employees, it is very important to take all kinds of precautions and to pay attention to the examination and treatment if necessary.
The COVID‐19 vaccine campaign in Turkey started on January 14, 2021. 11 As of 26 February, 2022, the first dose has been administered to 57 669 162 people, second dose to 52 787 534 people, and third dose to 26 993 218 people. 12 Several COVID‐19 vaccines in the world are in various stages of development. Currently, Pfizer‐BioNTech (mRNA‐based COVID‐19 vaccine), Sinovac (inactivated virus COVID‐19 vaccine), Sputnik V (adenovirus viral vector vaccine for COVID‐19), and TurkoVac (inactivated virus COVID‐19 vaccine) vaccines are used in Turkey. 13 , 14 Pfizer‐BioNTech and CoronaVac vaccines are currently being administered in the Siirt region. HCWs were among the first to be vaccinated against COVID‐19, but data on the vaccine's effectiveness among HCWs are still limited. This study aimed to compare Pfizer‐BioNTech and CoronaVac vaccine status and polymerase chain reaction (PCR) results in HCWs at our hospital. In addition, the relationship between the vaccination status of the employees and age, gender, length of stay, and laboratory results were examined.
2. METHODS
In our study, the COVID‐19 vaccination status of 1279 HCWs working at Siirt Training and Research Hospital based on whether they had COVID‐19 between July 15 and September 15, 2021 when the SARS‐CoV‐2 Delta variant was dominant, and the clinical course of the disease in those who had COVID‐19 were retrospectively examined. Since the HCWs in our hospital completed their third dose vaccine at the end of June 2021, the study was started on July 15, taking into account the 14‐day immunization period. The vaccination status of the employees was evaluated under three groups: fully vaccinated, incompletely vaccinated, and unvaccinated. Fully vaccinated and incompletely vaccinated groups were determined according to the protocols determined by the Republic of Turkey Ministry of Health at the time of the study. Those who received two doses of BioNTech or two doses of BioNTech +1 dose of CoronaVac or three doses of CoronaVac were considered to be fully vaccinated. Those with two doses of CoronaVac, one dose of BioNTech, one dose of CoronaVac, and one dose of CoronaVac+ one dose of BioNTech were each categorized as incompletely vaccinated. HCWs who have never been vaccinated are classified as unvaccinated. It was accepted that the protection of the vaccine started if at least 14 days had passed from the last dose of those who were fully vaccinated. At the time of the study, the protocol of the Ministry of Health did not include those who had COVID‐19 in the vaccination program, as it accepted that they were immune for 6 months. Therefore, those who had COVID‐19 in the 6 months before 15 July, 2021 were not included in the study.
Detection of SARS‐CoV‐2 in nasopharyngeal and oropharyngeal samples sent to the PCR laboratory for the diagnosis of COVID‐19 was carried out by the Real‐Time Reverse Transcriptase Polymerase Chain Reaction (RT‐PCR) method. RT‐PCR was performed with the Bio‐Speedy SARS‐CoV‐2 Emerging Plus detection kit (Bioeksen) on the CFX 96 Touch Real‐Time PCR instrument (Biorad). Threshold cycle numbers (C q) less than 33 in the FAM channel were considered positive. In patients whose blood values were measured, C‐reactive protein (CRP) level (normal value < 5 mg/L) was measured with AU 680 device (Beckman Coulter), and lymphocyte level (1.0−4.8 × 109/L normal reference range) was measured with BC‐6800 device (Mindray). Ferritin level (10−291 µg/L normal reference range) was measured by using Advia Centaur (Siemens), and d‐dimer level (normal level <540 µg/L) was measured by using Vidas fully automatic immune analyzer (Biomerieux).
In this study, the data were analyzed using the Statistical Package for the Social Sciences (SPSS) 26.0 statistical program. While evaluating the data, descriptive statistics were presented as the number of cases and percentages in categorical variables. In the analysis of continuous variables, normality analyzes were performed with the Kolmogorov−Smirnov goodness of fit test. Normally distributed data were compared with the analysis of variance (ANOVA) test, and data not showing were compared with the Kruskal−Wallis test. Comparisons of categorical data were made with the Χ 2 test. Means were presented with their standard deviations. Statistical significance was evaluated as p < 0.05.
This study was approved by Siirt University, Non‐invasive Clinical Research Ethics Committee (Decision NO:2021/11.01.05) the Republic of Turkey Ministry of Health.
3. RESULTS
A total of 1279 healthcare professionals, 863 (67.4%) men, and 416 (32.5%) women, were included in our study. The mean age of men is 37.0 ± 9.0 and the mean age of women is 31.8 ± 6.5. Considering the COVID‐19 vaccination status of HCW in our study, it was found that 590 (46.1%) were fully vaccinated, 403 (31.5%) were incompletely vaccinated, and 286 (22.4%) were unvaccinated. The mean age of the vaccine groups was 36 for those who were fully vaccinated, 32 for those who were incompletely vaccinated, and 31 for those who were unvaccinated, respectively. In the vaccine groups, the population and their mean age were higher in males than females. As can be seen in Table 1, a significant difference was found between the vaccine groups in the number of people, mean age, gender ratios, and mean age of the sexes (p < 0.05).
Table 1.
Number of people, mean age, gender ratios, and mean age of genders among vaccine groups.
| Completely vaccinated | Incompletely vaccinated | Unvaccinated | Test | P * | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Number of persons | 590 | 46.1 | 403 | 31.5 | 286 | 22.4 | Χ 2 | 0.000 (X 2 = 110.3) | |
| Mean of age** | 37.8 ± 8.9 | 36.0 | 33.7 ± 8.1 | 32.0 | 32.6 ± 7.2 | 31.0 | Kruskal−Wallis | 0.000 | |
| Gender | |||||||||
| Male | 445 | 34.8 | 251 | 19.6 | 167 | 13.1 | Χ 2 | 0.000 | |
| Female | 145 | 11.3 | 152 | 11.9 | 119 | 9.3 | |||
| Mean of age | |||||||||
| Male | 39.2 ± 9.0 | 38.0 | 35.1 ± 8.8 | 34.0 | 34.1 ± 8.0 | 32.0 | Kruskal−Wallis | 0.000 | |
| Female | 33.5 ± 7.1 | 32.0 | 31.3 ± 6.3 | 30.0 | 30.4 ± 5.5 | 29.0 | Kruskal−Wallis | 0.001 | |
p < 0.05 significant relationship, p > 0.05 no significant relationship.
In the measurement data, mean ± standard deviation was calculated instead of n, and median values were calculated instead of %.
It was observed that 20 (3.4%) of 590 fully vaccinated HCWs, 84 (20.8%) of 403 incompletely vaccinated HCWs, and 75 (26.2%) of 286 unvaccinated HCWs had COVID‐19 during the Delta variant dominant period (Table 2, Figure 1). When the COVID‐19 transmission rates of these three groups were examined in the period when the delta variant was dominant, a statistically significant difference was observed (p < 0.05). In addition, the rates of COVID‐19 transmission in the Delta variant dominant period of HCWs who were unvaccinated, vaccinated with two doses of BioNTech vaccines, and vaccinated with two doses of CoronaVac+ one dose of BioNTech vaccines were investigated. It was observed that 75 (26.2%) unvaccinated HWCs, 6 (4.4%) HCWs with two doses of BioNTech vaccine, 11 (2.7%) HCWs with two doses of CoronaVac+ one dose of BioNTech vaccines had COVID‐19 (Table 3, Figure 2).
Table 2.
The rates of coronavirus disease 2019 (COVID‐19) transmission in the Delta variant dominant period according to the vaccination status of healthcare workers.
| Vaccination status | No COVID‐19 (n = 1100) | Have had COVID‐19 (n = 179) | Test | p | X 2 | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Complete vaccinated | 570 | 96.6 | 20 | 3.4 | Χ 2 | 0.000 | 106.4 |
| Incomplete vaccinated | 319 | 79.2 | 84 | 20.8 | |||
| Unvaccinated | 211 | 73.8 | 75 | 26.2 | |||
Figure 1.
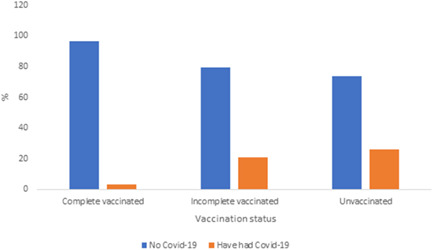
The rates of coronavirus disease 2019 (COVID‐19) transmission in the Delta variant dominant period according to the vaccination status of healthcare workers.
Table 3.
The rates of coronavirus disease 2019 (COVID‐19) transmission in the Delta variant dominant period according to different vaccination statuses of healthcare workers.
| Vaccination status | No COVID‐19 (N = 741) | Have had COVID‐19 (N = 92) | Test | p | X 2 | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Unvaccinated | 211 | 73.8 | 75 | 26.2 | Χ 2 | 0.000 | 102.5 |
| 2 BioNTech | 129 | 95.6 | 6 | 4.4 | |||
| 2 CoronaVac + 1 BioNTech | 401 | 97.3 | 11 | 2.7 | |||
Figure 2.
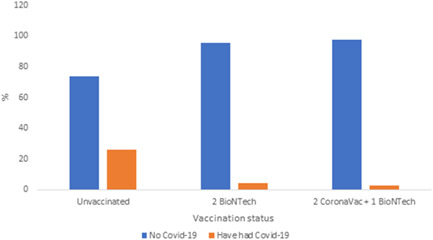
The rates of coronavirus disease 2019 (COVID‐19) transmission in the Delta variant dominant period according to different vaccination statuses of healthcare workers.
When the hospitalization rates and average days of hospitalization of those who had COVID‐19 in the period when the Delta variant was dominant were examined, hospitalization was not required in 20 (100%) of those who were fully vaccinated, 79 (94%) of those who were incompletely vaccinated, and 69 (92%) of those who were unvaccinated. While the need for intensive care was detected in one unvaccinated HCW, it was not seen in other vaccine groups. Among the healthcare professionals working in our hospital, the mean hospitalization days of COVID‐19 patients who were hospitalized were found to be 7.0 ± 3.7 in those with incomplete vaccination and 10.3 ± 8.6 in those who were unvaccinated (Table 4).
Table 4.
Hospitalization rates and mean days of hospitalization of those who had coronavirus disease 2019 (COVID‐19).
| Postvaccination COVID‐19 positive (N = 179) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Complete vaccinated (N = 20) | Incomplete vaccinated (N = 84) | Unvaccinated (N = 75) | Total | |||||
| n | % | n | % | n | % | n | % | |
| Hospitalization | ||||||||
| Yes | 0 a | 0.0 | 5 | 6.0 | 6 | 8.0 | 11 | 6.1 |
| No | 20 | 100 | 79 | 94.0 | 69 | 92.0 | 168 | 93.9 |
| Intensive care | ||||||||
| Yes | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 1 | 0.6 |
| No | 20 | 100 | 84 | 100 | 74 | 98.7 | 178 | 99.4 |
| Hospitalization | Complete vaccinated (N = 0) | Incomplete vaccinated (N = 5) | Unvaccinated (N = 6) | Total | ||||
| n | % | n | % | n | % | n | % | |
|---|---|---|---|---|---|---|---|---|
| Mean of hospitalization dayb | 0 | 0 | 7.0 ± 3.7 | 7.0 | 10.3 ± 8.6 | 8.5 | 8.8 ± 6.7 | 7.0 |
Statistical analysis was not performed as the situation was stable.
In the measurement data, mean ± standard deviation was calculated instead of n, and median values were calculated instead of %.
After being examined by specialist physicians, patients who apply to our hospital's pandemic outpatient clinic are subjected to computed tomography (CT) to detect lung involvement, if deemed necessary, according to a standardized procedure according to the algorithms of the Ministry of Health, and blood values giving information about prognosis, such as CRP, lymphocyte, ferritin, and d‐dimer are requested. In our study, CT reports, blood values, if any, of HCWs who had COVID‐19 in the Delta variant dominant period were examined and a comparison was made between groups according to their vaccination status. The blood tests were requested from 10% of fully vaccinated HCWs who had COVID‐19, 19% of those who were incompletely vaccinated, and 21.3% of those who were unvaccinated (Table 5). The results of CRP, lymphocyte, ferritin, and d‐dimer, which are considered prognostic factors in patients with COVID‐19, were compared and are shown in Table 6. When these blood values were compared, a significant difference was found between the groups only for lymphocytes (p = 0.041). Fully vaccinated patients had a higher lymphocyte count than incompletely vaccinated and unvaccinated patients. Although CRP, d‐dimer, and ferritin, which are used as prognostic factors, were found to be higher in unvaccinated and incompletely vaccinated patients compared to fully vaccinated patients, they were not found to be statistically significant (p > 0.05).
Table 5.
The rates of requesting blood values of those who had coronavirus disease 2019 (COVID‐19) in the Delta variant dominant period.
| Postvaccination COVID‐19 positive (N = 179) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Completely vaccinated (N = 20) | Incompletely vaccinated (N = 84) | Unvaccinated (N = 75) | Total | ||||||
| n | % | n | % | n | % | n | % | ||
| Blood Collectiona | Yes | 2 | (10.0) | 16 | (19.0) | 16 | (21.3) | 34 | (19.0) |
| No | 18 | (90.0) | 68 | (81.0) | 59 | (78.7) | 145 | (81.0) | |
Statistical analysis could not be performed because the values did not meet the Χ 2 test criteria.
Table 6.
Comparison of the prognostic blood values of those who had coronavirus disease 2019 (COVID‐19) in the Delta variant dominant period.
| Patients who have taken blood (N = 34) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Completely vaccinated (N = 2) | Incompletely vaccinated (N = 16) | Unvaccinated (N = 16) | Test | p. | |||||||
| Mean ± standard deviation | Min | Max | Mean ± standard deviation | Min | Max | Mean ± standard deviation | Min | Max | |||
| CRP* | 16.1 ± 2.3 | 14.5 | 17.7 | 23.4 ± 24.2 | 2.5 | 96.1 | 30.2 ± 37.2 | 0.8 | 106.3 | Kruskal−Wallis | 0.952 |
| Lymphocyte * | 4.3 ± 2.3 | 2.7 | 5.9 | 2.1 ± 1.1 | 0.8 | 4.2 | 1.7 ± 1.5 | 0.4 | 6.7 | Kruskal−Wallis | 0.041 |
| Ferritin * | 148.1 ± 196.5 | 18.6 | 1146.0 | 189.1 ± 281.0 | 18.6 | 1146.0 | 482.3 ± 676.8 | 6.9 | 1650.0 | Kruskal−Wallis | 0.8380 |
| d‐Dimer* | 189.5 ± 141.6 | 89.3 | 289.6 | 427.4 ± 293.6 | 112.1 | 869.8 | 779.2 ± 1095.8 | 54.6 | 3886.3 | Kruskal−Wallis | 0.574 |
*p < 0.05 significant relationship, p > 0.05 no significant relationship.
In our study, oxygen support status and CT uptake rates were examined to compare the severity of lung involvement of those who had COVID‐19 in the SARS‐CoV‐2 Delta variant dominant period. None of those who were fully vaccinated and had COVID‐19 needed oxygen support. Oxygen support was provided by mask to 2 (2.4%) persons and nasally to 3 (3.6%) of those who were incompletely vaccinated. Among the unvaccinated, 2 (2.7%) HCWs had to be given oxygen in the form of Hı‐flow, and 4 (5.3%) HCWs had to be given oxygen by mask. CT was not required in 95% of those who were vaccinated, 86.9% of those who were incompletely vaccinated, and 87.2% of those who were unvaccinated. While severe lung involvement was not observed in fully vaccinated and incompletely vaccinated patients, it was found only in 3 (4%) unvaccinated patients (Table 7).
Table 7.
Oxygen support status and computed tomography (CT) uptake rates of those who had COVID‐19 in the Delta variant dominant period.
| Postvaccination COVID‐19 positive (N = 179) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Completely vaccinated (N = 20) | Incompletely vaccinated (N = 84) | Unvaccinated (N = 75) | Total | ||||||
| n | % | n | % | n | % | n | % | ||
| Oxygen demand | HI‐FLOW | 0 a | (0.0) | 0 | (0.0) | 2 | (2.7) | 2 | (1.1) |
| Mask | 0 | (0.0) | 2 | (2.4) | 4 | (5.3) | 6 | (3.4) | |
| Nasal | 0 | (0.0) | 3 | (3.6) | 0 | (0.0) | 6 | (1.7) | |
| Not | 20 | (100) | 79 | (94.0) | 69 | (92.0) | 168 | (93.9) | |
| CT | Serious | 0 | (0.0) | 0 | (0.0) | 3 | (4.0) | 3 | (1.7) |
| Mild | 0 | (0.0) | 5 | (6.0) | 2 | (2.7) | 7 | (3.9) | |
| Normal | 0 | (0.0) | 3 | (3.6) | 4 | (5.3) | 8 | (4.5) | |
| Moderate | 1 | (5.0) | 3 | (3.6) | 2 | (2.7) | 5 | (2.8) | |
| Not | 19 | (95.0) | 73 | (86.9) | 64 | (85.3) | 156 | (87.2) | |
Since the situation was stable, statistical analysis was not performed.
4. DISCUSSION
BNT162b2 COVID‐19 vaccine developed by Pfizer and BioNTech has been reported to have 95% efficacy in preventing symptomatic SARS‐CoV‐2 infection in Phase 3, a placebo‐controlled randomized clinical trial. 15 It was the first COVID‐19 vaccine to receive an emergency use authorization by the US Food and Drug Administration. 16 The mRNA vaccine effectiveness of full immunization against COVID‐19 was 90% regardless of symptoms in a prospective cohort of 3950 HCWs weekly tested for SARS‐CoV‐2 for 13 weeks under real‐world conditions. Furthermore, after the first dose, vaccine effectiveness was at 80%. 9 In recent studies, Daniel et al., 17 in their study at the University of Texas Southwestern Medical Center, presented early reports on the effect of mRNA vaccines BNT162b2 vaccine (Pfizer‐BioNTech) and mRNA‐1273 (Moderna) on hospital employees. They reported that 234 of 8969 (2.61%) unvaccinated employees, 112 of 6144 (1.82%) partially vaccinated employees, and 4 of 8121 fully (0.05%) vaccinated employees were infected. Ioannou et al. 4 studied the transmission of SARS‐CoV‐2 variant B.1.1.7 among HCWs vaccinated with at least one dose of BNT162b2 mRNA vaccine. They reported that of 55 PCR‐positive HCWs, 24 (44%) received at least one dose of the BNT162b2 vaccine, and 21 were fully vaccinated (diagnosed with COVID‐19 >2 weeks after the second dose). At one of the largest medical centers in Israel, Bergwerk et al. 18 investigated SARS‐CoV‐2 breakthrough infections in HCWs who were fully vaccinated with the BNT162b2 mRNA vaccine. They documented 39 SARS‐CoV‐2 breakthrough infections among 1497 fully vaccinated HCWs. In a study of 6710 HCWs, of whom 5953 (88.7%) received at least one dose, 5517 (82.2%) received two doses of BNT162b2 vaccine, and 757 (11.3%) received no vaccine, symptomatic SARS‐CoV‐2 infection was reported to occur in 8 fully vaccinated and 38 unvaccinated HCWs. They also reported that asymptomatic SARS‐CoV‐2 infection was detected in 19 fully vaccinated and 17 unvaccinated HCWs. 16 CoronaVac was the first SARS‐CoV‐2 vaccine to be administered in Turkey on January 14, 2021. Vaccination with the BNT162b2 vaccine began in April 2021. In the Phase 3 trial in Turkey, the CoronaVac vaccine was reported as 83.5% effective in preventing PCR‐confirmed symptomatic COVID‐19 infection. 19 Azap et al., 20 in a study on the effectiveness of the CoronaVac vaccine on HCWs in Turkey, reported that 2173 (60.3%) of 3600 HCWs were vaccinated with two doses of CoronaVac. They reported that 62 of them were positive for SARS‐CoV‐2 in PCR tests. It was determined that 39 (62.9%) of these 62 HCWs were fully vaccinated, 20 (32.2%) were unvaccinated, and 3 (4.8%) had a single‐dose vaccination. As a result of Phase 1−2 trials of the CoronaVac vaccine conducted in China among participants aged 18−59 years 21 and participants aged 60 years and older, 22 In most patients, 14 days after receiving the second dose, the vaccine was reported to be safe and immunogenic. Preliminary estimates from an observational study involving vaccinated HCWs (from a preprint server) have suggested that at least one dose of the CoronaVac vaccine has been 49.6% effective against COVID‐19 in Manaus, Brazil, a place where P.1 (or gamma) variant is common. It was reported that 776 (28%) of the 2797 HCWs who received at least one dose of the CoronaVac vaccine had positive RT‐PCR test results. 23 In our study, a significant difference was observed between the fully vaccinated, incompletely vaccinated, and unvaccinated groups in terms of COVID‐19 transmission rates (p < 0.05). While the rate of COVID‐19 transmission was lowest in fully vaccinated HCWs (3.4%), the rate of transmission was highest in those who were unvaccinated (26.2%). A statistically significant difference was found between COVID‐19 transmission rates in unvaccinated HCWs and HCWs vaccinated with two doses of BioNTech vaccines and two doses of CoronaVac+ one dose of BioNTech vaccines (p < 0.05). The rate of COVID‐19 transmission in HCWs with two doses of BioNTech vaccine (4.4%) and two doses of CoronaVac+ one dose of BioNTech vaccines (2.7%) was found to be quite low compared to unvaccinated (26.2%). The lowest infection rate was found in the workers who received two doses of CoronaVac+ one dose of BioNTech (2.7%). In the current study, hospitalization was not required in fully vaccinated HCWs. Ioannou et al. 4 reported that only two HCWs who were not vaccinated needed to be hospitalized. In a recent study, Tenforde et al. 24 reported that vaccination with an mRNA COVID‐19 vaccine was significantly lower among patients hospitalized with COVID‐19 whose disease progressed to death or had invasive mechanical ventilation.
Lymphopenia and elevations of d‐dimer, ferritin, interleukin 6 (IL‐6), troponin and myoglobin, CRP, and lactate dehydrogenase were described in early reports as predictive biomarkers for the clinical outcome of hospitalized patients. 25 In our study, CRP, lymphocyte, ferritin, and d‐dimer level were measured and used as prognostic factors. Only lymphocytes (p = 0.041) showed a significant difference between the groups when these blood values were compared. There are many reports of a decrease in the absolute lymphocyte count (lymphopenia) in severe cases of COVID‐19. 26 , 27 , 28 In the current study, lymphocyte count was found to be significantly higher in fully vaccinated patients compared to incompletely vaccinated and unvaccinated patients. In previous studies, an increase in CRP, ferritin, and d‐dimer was reported in COVID‐19 patients. 28 , 29 , 30 Although prognostic factors, such as CRP, d‐dimer, and ferritin were found to be higher in unvaccinated and partially vaccinated patients compared to fully vaccinated patients, the differences were not statistically significant (p > 0.05). The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend oxygen therapy as the first‐line treatment for COVID‐19‐induced respiratory distress and hypoxia. Administration methods differ and should be determined by the severity of the illness. The goal of treatment should be to keep oxygen saturation above 90%. 31 In our study, none of the fully vaccinated patients who had COVID‐19 needed oxygen support. However, oxygen was given to 2.7% of the unvaccinated patients through Hi‐Flow, and 5.3% by mask.
Three different situations limited our study. First, because our study was conducted in a single center, the number of participants was relatively small and the protection period of the vaccines was followed for a short period of 3 months. Conducting more comprehensive and longer‐term studies on this subject may affect the results. The second is that the comorbid conditions that may affect the prognosis of the COVID‐19 patients in our study are not known. Third, we did not assess the impact on vaccine efficacy of those who had COVID‐19 more than 6 months before September 15, 2021.
In summary, the rate of COVID‐19 transmission was lowest in fully vaccinated HCWs and the transmission rate was lowest among those vaccinated with two doses of CoronaVac+ one dose of BioNTech. Hospitalization was not required in fully vaccinated HCWs. We can conclude that full vaccination significantly reduces the rate of contracting COVID‐19 and disease severity compared to those who are not vaccinated.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Özüdoğru O, Acer Ö, Genç Bahçe Y. Risks of catching COVID‐19 according to vaccination status of healthcare workers during the SARS‐CoV‐2 Delta variant dominant period and their clinical characteristics. J Med Virol. 2022;94:3706‐3713. 10.1002/jmv.27778
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. WHO . WHO Coronavirus (COVID‐19). 2022. Accessed March 11, 2022. https://covid19whoint/
- 2. WHO . Turkey: WHO coronavirus (Covid‐19) disease dashboard. 2022. Accessed March 17, 2022. https://covid19.who.int/region/euro/country/tr
- 3. Hall VJ, Foulkes S, Saei A, et al. COVID‐19 vaccine coverage in health‐care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. The Lancet. 2021;397(10286):1725‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ioannou P, Karakonstantis S, Astrinaki E, et al. Transmission of SARS‐CoV‐2 variant B. 1.1. 7 among vaccinated health care workers. Infect Dis. 2021;53(11):876‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forni G, Mantovani A. COVID‐19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N Engl J Med. 2021;385(10):875‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasileiou E, Simpson CR, Shi T, et al. Effectiveness of first dose of COVID‐19 vaccines against hospital admissions in Scotland: national prospective cohort study of 5.4 million people. Lancet (preprint). 2021;397:1646‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA‐1273 COVID‐19 vaccines in preventing SARS‐CoV‐2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb Mortal Wkly Rep. 2021;70(13):495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer‐BioNTech COVID‐19 vaccine among residents of two skilled nursing facilities experiencing COVID‐19 outbreaks—Connecticut, December 2020–February 2021. Morb Mortal Wkly Rep. 2021;70(11):396‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xinhua News Agency . Turkey begins mass COVID‐19 vaccination with Sinovac's jabs. 2021. Accessed February 11, 2022. http://www.xinhuanet.com/english/2021-01/14/c_139668037_4.htm
- 12.COVID‐19 vaccination information platform. 2022. https://covid19asi.saglik.gov.tr/?_Dil=2
- 13. Anadolu Agency . Turkey OKs emergency use of Sputnik‐V vaccine. 2021.
- 14. Anadolu Agency .1st Doses of Pfizer‐BioNTech Jab Administered in Turkey. 2021. [Google Scholar]
- 15. Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S. C4591001 clinical trial group: safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS‐CoV‐2 infections among health care workers. JAMA. 2021;325(24):2457‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS‐CoV‐2 vaccine at one medical center. N Engl J Med. 2021;384(20):1962‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergwerk M, Gonen T, Lustig Y, et al. COVID‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azap OK, Arslan H, Erol C, Yalcin TY, Sari N. Healthcare workers should be recieved with the highest effective vaccine available. Infect Dis Clin Microbiol. 2021;3(2):107‐109. [Google Scholar]
- 21. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18−59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hitchings MD, Ranzani OT, Torres MSS, et al. Effectiveness of CoronaVac in the setting of high SARS‐CoV‐2 P. 1 variant transmission in Brazil: a test‐negative case‐control study. medRxiv . 2021. 10.1101/2021.04.07.21255081 [DOI] [PMC free article] [PubMed]
- 24. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID‐19 hospitalization and disease severity. JAMA. 2021;326(20):2043‐2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID‐19 infection. Clin Infect Dis. 2020;71(8):1962‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghayda RA, Lee J, Lee JY, et al. Correlations of clinical and laboratory characteristics of COVID‐19: a systematic review and meta‐analysis. Int J Environ Res Public Health. 2020;17(14):5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Para O, Caruso L, Pestelli G, et al. Ferritin as prognostic marker in COVID‐19: the FerVid study. Postgrad Med. 2022: 58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C‐reactive protein, procalcitonin, D‐dimer, and ferritin in severe coronavirus disease‐2019: a meta‐analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whittle JS, Pavlov I, Sacchetti AD, Atwood C, Rosenberg MS. Respiratory support for adult patients with COVID‐19. JACEP Open. 2020;1(2):95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


