Abstract
Objective
Olfactory dysfunction (OD) is a common presenting symptom of COVID‐19 infection. Radiological imaging of the olfactory structures in patients with COVID‐19 and OD can potentially shed light on its pathogenesis, and guide clinicians in prognostication and intervention.
Methods
PubMed, Embase, Cochrane, SCOPUS were searched from inception to August 1, 2021. Three reviewers selected observational studies, case series, and case reports reporting radiological changes in the olfactory structures, detected on magnetic resonance imaging, computed tomography, or other imaging modalities, in patients aged ≥18 years with COVID‐19 infection and OD, following preferred reporting items for systematic reviews and meta‐analyses guidelines and a PROSPERO‐registered protocol (CRD42021275211). We described the proportion of radiological outcomes, and used random‐effects meta‐analyses to pool the prevalence of olfactory cleft opacification, olfactory bulb signal abnormalities, and olfactory mucosa abnormalities in patients with and without COVID‐19‐associated OD.
Results
We included 7 case–control studies (N = 353), 11 case series (N = 154), and 12 case reports (N = 12). The pooled prevalence of olfactory cleft opacification in patients with COVID‐19 infection and OD (63%, 95% CI = 0.38–0.82) was significantly higher than that in controls (4%, 95% CI = 0.01–0.13). Conversely, similar proportions of cases and controls demonstrated olfactory bulb signal abnormalities (88% and 94%) and olfactory mucosa abnormalities (2% and 0%). Descriptive analysis found that 55.6% and 43.5% of patients with COVID‐19 infection and OD had morphological abnormalities of the olfactory bulb and olfactory nerve, respectively, while 60.0% had abnormal olfactory bulb volumes.
Conclusion
Our findings implicate a conductive mechanism of OD, localized to the olfactory cleft, in approximately half of the affected COVID‐19 patients. Laryngoscope, 132:1260–1274, 2022
Keywords: anosmia, COVID‐19, imaging, olfactory, radiology, SARS‐CoV‐2, smell
INTRODUCTION
Acute olfactory dysfunction (OD) may be one of the earliest presenting symptoms of coronavirus disease 19 (COVID‐19) infection, which has been reported in 34%–68% of symptomatic patients. 1 In the context of COVID‐19 infection, acute OD is defined as decreased or altered sense of smell of a duration of 14 days or less, in the absence of chronic rhinosinusitis, a history of head trauma, or neurotoxic medications. 2
While the pathogenesis of OD in COVID‐19 is not fully understood, studies have found that nasal epithelial and sustentacular cells of the olfactory epithelium demonstrate high expression of the angiotensin‐converting enzyme 2 receptor required for entry of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus. 2 , 3 Disruption of olfactory neuroepithelial cells may result in inflammation, causing subsequent olfactory receptor neuron damage and impairment of neurogenesis. 4 Other studies postulate that the SARS‐CoV‐2 virus ascends through the olfactory cleft, cribriform lamina, olfactory bulb, and olfactory nerve pathway to cause direct central nervous system effects. 5 , 6 , 7
COVID‐19‐associated OD has clinical significance, with possible implications on long‐term cognitive outcomes. While one study has hypothesized that COVID‐19‐associated OD may confer in ApoE4 carriers an increased risk of future dementia owing to virus‐induced chronic modifications in the central nervous system, 8 another has demonstrated that hyposmia may represent a useful clinical biomarker for both neurological involvement and cognitive impairment in mild COVID‐19 infection. 9
Computed tomography (CT) and magnetic resonance imaging (MRI) are valuable methods of evaluating the olfactory structures in patients presenting with OD, 10 , 11 with the ability to discriminate among various etiologies, and guide prognostication of clinical outcomes. 11 Abnormal radiological findings of the olfactory structures have been extensively studied in other conditions associated with OD. For instance, in chronic rhinosinusitis, the inflammatory status of the olfactory cleft on imaging has been found to be strongly correlated with the degree of olfactory loss. 12 Structural imaging has also been used to qualify damage to the central olfactory structures, including volumetric reduction of the olfactory bulbs and olfactory cortex, which may account for OD in mild cognitive impairment and Alzheimer's disease. 13
In current practice, olfactory imaging is not routinely performed for COVID‐19 patients with OD. However, neuroimaging abnormalities related to COVID‐19‐associated OD have been gaining attention, including abnormalities of the olfactory bulb, olfactory sulcus, olfactory cleft, and olfactory tract on imaging. 14 , 15 These imaging findings may shed light on the mechanisms underlying COVID‐19‐associated OD, 16 offering insight into the route of entry of the SARS‐CoV‐2 virus, and involvement of anatomical structures in the brain and olfactory pathways. 17 Moreover, knowledge gained from such data may enable clinicians to more accurately predict the clinical course, and develop targeted interventions to treat COVID‐19‐associated OD. 18
To our knowledge, there has not yet been any study collectively examining the current literature surrounding this topic. Hence, we conducted a systematic review and meta‐analysis to evaluate the radiological changes in the olfactory structures in COVID‐19 patients with OD.
METHODS
Search Algorithm
This systematic review was registered on PROSPERO (CRD42021275211), and conducted in accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) 2020 guidelines. 19 Searches of four databases (PubMed, Embase, Cochrane, and SCOPUS) were conducted for articles published from date of inception to August 1, 2021, using search terms for COVID‐19, olfactory dysfunction, olfactory structures, and imaging modalities (full search strategy available in Supplemental Methods). The PRISMA checklist is included in Table S1.
Study Selection
In accordance with the Population Intervention Comparison and Outcome criteria framework, articles were included if they met the following criteria: observational studies, case series, and case reports reporting radiological changes in the olfactory structures, detected on MRI, CT, or positron emission tomography (PET), in patients aged 18 years and above with COVID‐19 infection and OD, compared with radiological findings in control subjects where possible. Control subjects included normosmic individuals with COVID‐19 infection and normosmic individuals without COVID‐19 infection. Non‐English articles or articles without English translation were excluded. Reviews, letters, conference abstracts, or other records not published as full‐length articles in peer‐reviewed journals were also excluded.
Three reviewers (C.J.W.T., H.T.L., X.Y.T.) independently selected eligible studies (based on title and abstract, followed by full‐text articles). Any disagreement was resolved by discussion with a fourth author (B.K.J.T.). We extracted key data from each included article (Supplemental Methods).
Quality control was performed by using the Newcastle–Ottawa Scale, 20 acknowledged by the Cochrane Collaboration 21 (Table S2). The maximum total score was 9. As per the newcastle‐ottawa scale grading in past reviews, we graded studies as having a high (<5 stars), moderate (5–7 stars), or low risk of bias (≥8 stars). 22
Statistical Analysis
All analyses were conducted using RevMan (version 5.4) and R (version 4.0.3), in accordance with statistical approaches laid out by the Cochrane handbook. A two‐sided p value <0.05 was considered statistically significant. Sufficient information was found in the literature to pool the proportions of patients with olfactory cleft opacification, olfactory mucosa abnormalities, and olfactory bulb signal abnormalities in meta‐analyses. The Q‐test or the I 2 statistic was used to assess between‐study heterogeneity. 23 There were insufficient studies and hence insufficient statistical power to assess potential sources of between‐study heterogeneity via meta‐regression, 21 , 24 or to assess publication bias via visual inspection of funnel plot asymmetry, Egger's bias, or trim‐and‐fill. Descriptive analysis of overall percentages was used for other outcomes involving the olfactory bulbs, olfactory clefts, olfactory tracts, olfactory sulci, olfactory gyri, olfactory nerves, olfactory cortex, cribriform plates, and paranasal sinuses, where aggregate data were insufficient for meta‐analysis.
RESULTS
The PRISMA flowchart demonstrating the study selection process is presented in Figure 1. Literature search of the four databases (PubMed, Embase, Cochrane, and SCOPUS) retrieved 537 results. Fifteen duplicates were removed. Title and abstract screening further excluded 453 articles. Full‐text screening excluded 39 articles. Thirty articles were included in the final analysis.
Fig. 1.
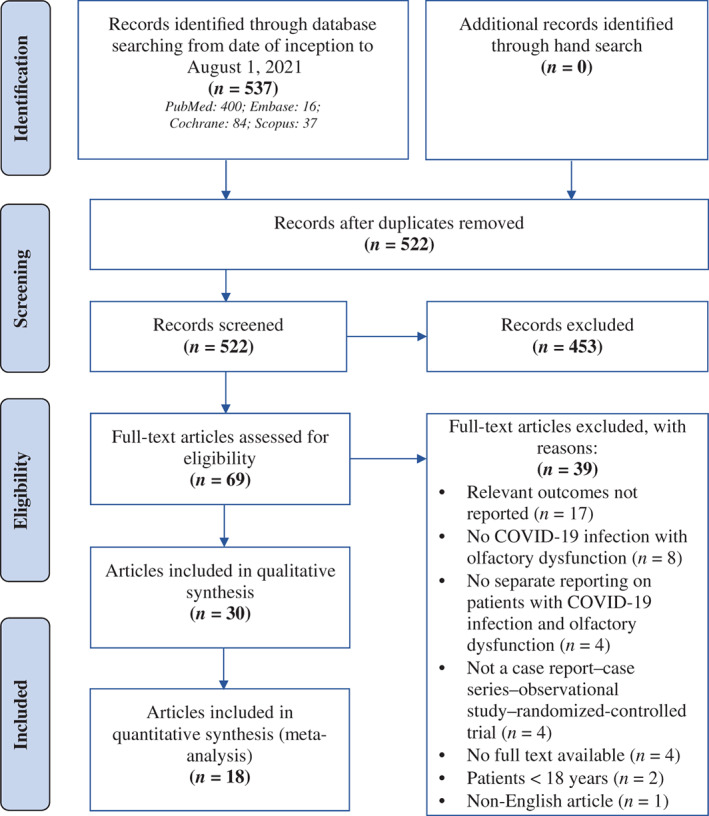
Preferred reporting items for systematic reviews and meta‐analyses flow diagram summary of study selection process. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Study Characteristics
Study characteristics for aggregate studies and individual case reports are summarized in Tables I, II, III, and S3–S6. Briefly, there were 7 case–control studies (N = 353), 25 , 26 , 27 , 28 , 29 , 30 , 31 11 case series (N = 154), 16 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 and 12 case reports (N = 12), 17 , 18 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 with a total of 30 included studies (N = 518). Of these 518 individuals, 292 had COVID‐19 infection with OD (56.4%) while the remaining 226 were controls (43.6%). Among the control group, 178 individuals were healthy individuals without COVID‐19 infection (34.4%), 10 were individuals with COVID‐19 infection without OD (1.9%), and 38 were individuals with anosmia due to viral illnesses other than COVID‐19 (7.4%). The percentage of males in the included aggregate studies ranged from 25% to 60.5%, while mean age ranged from 34.3 to 45.4 years. Case–control study sample sizes ranged from 16 to 91. MRI, CT, and PET were utilized in 26, 6, and 5 studies, respectively.
TABLE I.
Baseline Characteristics for Aggregate Studies (Demographics).
| Study | No. of Individuals | Age (years) | Percentage of Males (%) | Study Group | Control Group | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | IQR | |||||
| Güney (2021) | 83 |
Study group: 40.27 ± 14.5 Control group: 40.27 ± 14.4 |
Study group: 48.8 Control group: 47.6 |
41 individuals with COVID‐19 and OD | 42 adult healthy individuals proven to be free from COVID‐19 infection by PCR test and serum antibody test | ||
| Lechien (2020) | 16 | 36 ± 10.1 | 50 | 16 individuals with COVID‐19 and OD | Nil | ||
| Eliezer (2020) | 40 |
Study group: 34.6 ± 8.8 Control group: 33.9 ± 7.8 |
55 | 20 individuals with COVID‐19 and OD | 20 age‐matched control healthy individuals | ||
| Chetrit (2020) | 23 | 39.0 ± 17.1 | 39.1 | 19 individuals with COVID‐19 and OD | 4 individuals with COVID‐19 without OD | ||
| Shor (2021) | 20 | NR | NR | 10 individuals with COVID‐19 and OD | 10 age‐ and sex‐matched healthy individuals | ||
| Chung (2021) | 4 | NR | 50 | 2 individuals with COVID‐19 and OD | 2 healthy individuals who negative for COVID‐19 2 by PCR assays at time of MRI evaluation | ||
| Tsivgoulis (2021) | 16 | 45.4 ± 12.4 | 25 | 8 individuals with COVID‐19 and OD | 8 age‐ and sex‐matched healthy individuals | ||
| Kandemirli (2021) | 23 | 29 | 22–41 | 39.1 | 23 individuals with COVID‐19 and OD | Nil | |
| Altundag (2021) | 91 |
39.3 ± 12 Study group: 35 ± 11.5 Control group: 43.7 ± 11.8 (OD due to viral infection other than COVID‐19), 36.9 ± 11 (healthy controls) |
47.3 Study group: 11.0 Control group: 18.7 (OD due to viral infection other than COVID‐19), 17.6 (healthy controls) |
24 individuals with COVID‐19 and OD | 29 healthy individuals who underwent paranasal sinus CT due to headache or tinnitus and Sniffin' Sticks olfactory test; 38 individuals with anosmia due to viral infection other than COVID‐19 with URI immediately prior to OD, OD persisting for at least 8 weeks, pathological findings on Sniffin' Sticks olfactory test, and CT scan images at time of olfactory evaluation, without history of trauma, sinonasal surgery, evidence of sinonasal inflammation on nasal endoscopy or paranasal sinus CT scan | ||
| Yıldız (2021) | 80 |
Study group: 47.58 ± 20.57 Control group: 41.27 ± 18.27 |
50 | 40 individuals with COVID‐19 and OD | 40 healthy individuals without COVID‐19 who applied to the clinic with a headache, and therefore, underwent paranasal sinus CT scan | ||
| Naeini (2020) | 49 | 45.08 ± 12.2 | 44.9 | 49 individuals with COVID‐19 and OD | Nil | ||
| Niesen (2021) | 38 | 42.6 | 60.5 | 12 individuals with COVID‐19 and OD | 26 healthy individuals without any history of COVID‐19, smell or taste disorders | ||
CT = computed tomography; MRI = magnetic resonance imaging; NR = not reported; OD = olfactory dysfunction; PCR = polymerase chain reaction.
TABLE II.
Baseline Characteristics for Aggregate Studies (Comorbidities and Symptoms).
| Study | Comorbidities | Other Symptoms in Study Group | COVID‐19 Diagnosis | ||
|---|---|---|---|---|---|
| Study Group | Control Group | COVID‐19 Symptoms | Rhinologic Symptoms | ||
| Güney (2021) | NR | NR | NR | NR | RT‐PCR (stick test) |
| Lechien (2020) | NR | NR | NR | Mild nasal obstruction (n = 5); Moderate nasal obstruction (n = 1) | RT‐PCR (nasopharyngeal swab) |
| Eliezer (2020) | NR | NR | Fever (n = 6); Myalgia (n = 16); dyspnea (n = 2); dysgeusia (n = 17) | Cough (n = 11) | RT‐PCR |
| Chetrit (2020) | Hypertension (n = 2); depression (n = 2); asthma (n = 2); hypothyroidism (n = 1); diabetes (n = 1); heart problems (n = 1); neurological diseases (n = 1); autoimmune disease (n = 1); hypercholesterolemia (n = 1) | NR | Asthenia (n = 21); headache (n = 18); fever (n = 15); myalgia (n = 14); loss of appetite (n = 12); arthralgia (n = 10); chest pain (n = 9); dyspnea (n = 5); diarrhea (n = 5); abdominal pain (n = 2); conjunctivitis (n = 2); dysphagia (n = 2); taste dysfunction (n = 21); throat sputum (n = 12); sore throat (n = 6) | Cough (n = 17); postnasal drip (n = 21); nasal obstruction (n = 18); ear pain (n = 18); rhinorrhoea (n = 17); face pain or heaviness (n = 16) | Laboratory‐confirmed |
| Shor (2021) | NR | NR | NR | NR | NR |
| chung (2021) | NR | NR | NR | NR | RT‐PCR (nasopharyngeal and throat swab) |
| Tsivgoulis (2021) | Hypertension (n = 1); hypothyroidism (n = 5); allergic rhinitis (n = 3); dyslipidemia (n = 1); depression (n = 1); rhinoplasty (n = 1); iron deficiency anemia (n = 2); Helicobacter pylori gastritis (n = 1) | NR | Fever (n = 5); ageusia (n = 7); headache (n = 2); myalgia (n = 2); fatigue (n = 4) | Coryza (n = 1) | RT‐PCR |
| Kandemirli (2021) | NR | NR | Rhinorrhoea and/or nasal obstruction (n = 7) | RT‐PCR (swab) | |
| Altundag (2021) | NR | NR | NR | NR | RT‐PCR from nasal and nasopharyngeal swabs |
| Yıldız (2021) | None (n = 12); hypertension (n = 11); asthma (n = 1); COPD (n = 3); diabetes mellitus (n = 9); Alzheimer's (n = 1); epilepsy (n = 1); heart failure (n = 1); multiple sclerosis (n = 1) | None (n = 27); hypertension (n = 8); asthma (n = 1); diabetes mellitus (n = 2); heart failure (n = 2) | Fever (n = 22); headache (n = 28); dyspnea (n = 26); myalgia (n = 19); diarrhea or abdominal pain (n = 3) | Cough (n = 26) | RT‐PCR (nasopharyngeal swab) |
| Naeini (2020) | Hypothyroidism (n = 5); diabetes mellitus (n = 13); hypertension (n = 10; asthma (n = 6) | NR | Fever (n = 41); halitosis (n = 2); headache (n = 35); ear pain (n = 4); dental pain (n = 1); fatigue (n = 31); sore throat (n = 22); dyspnea (n = 32); dysgeusia (n = 37) | Cough (n = 31); rhinorrhoea (n = 9); sneezing (n = 7); nasal obstruction (n = 16); purulent discharge (n = 8); facial fullness (n = 7); facial pain (n = 12) | RT‐PCR (pharyngeal swab) |
| Niesen (2021) | Respiratory allergy (n = 6); asthma (n = 1); hypertension (n = 2); hypothyroidism (n = 1) | NR | Dysgeusia (n = 11); myalgia or arthralgia (n = 10); fever (n = 6); dyspnea (n = 5); asthenia (n = 5); diarrhea (n = 3); nausea and/or vomiting (n = 2); rash (n = 1); throat pain (n = 1); headache: (n = 6) | Cough (n = 8); rhinorrhoea (n = 8) | Nasopharyngeal swabs with direct antigen detection in 3 individuals and RT‐PCR in 9 individuals |
NR = not reported; RT‐PCR = reverse transcription‐polymerase chain reaction.
TABLE III.
Baseline Characteristics for Aggregate Studies (Diagnosis and Assessment of Olfactory Dysfunction).
| Study | Type of OD | Olfactory Dysfunction Diagnosis | Objective Olfactory Assessment Scores | Duration of OD (days) | |
|---|---|---|---|---|---|
| Study Group | Control Group | ||||
| Güney (2021) | Hyposmia (n = 38); anosmia (n = 3) | Visual analog scale (VAS) | Mean VAS: 3.39 ± 1.46 | NR | NR |
| Lechien (2020) | Anosmia (n = 16) | Sinonasal outcome test 22 (SNOT‐22); Questionnaire of Olfactory Disorders‐Negative Statements (sQOD‐NS); Sniffin' Sticks score | Mean Sniffin' Sticks score: 4.6 ± 1.7 | NR | NR |
| Eliezer (2020) | Not specified | Visual olfactive score (VOS) |
VOS—mean: 1.6 ± 1.9; range: 0–6 Olfactory score—mean: 2.8 ± 2.7; range: 0–8 |
Olfactory score—mean: 9.4 ± 0.7; range: 8–10 | NR |
| Chetrit (2020) | Hyposmia (n = 19); anosmia (n = 4) | National Health and Nutrition Examination Survey (NHNES)—smell and taste component; Sniffin' Sticks tests | Mean Sniffin' Sticks score: 3.2 ± 4.4 | 9.6 ± 6.9 | |
| Shor (2021) | Anosmia (n = 10) | NR | NR | NR | NR |
| Chung (2021) | Anosmia (n = 2) | Sinonasal outcome test (SNOT‐22); butanol threshold test (BTT); smell identification test (SIT) | NR | NR | NR |
| Tsivgoulis (2021) | Microsmia (n = 6); anosmia (n = 2) | Self‐reporting; three‐odorant test Quick Smell Identification TestTM (Q‐SITTM); sinonasal outcome test 22 (SNOT 22) questionnaire | NR | NR | NR |
| Kandemirli (2021) | Anosmia (n = 23) | Sniffin' Sticks test battery | Median threshold score: 1 (IQR 1–2.25); median discrimination score: 2 (IQR 0–3); median identification score: 2 (IQR 0–4); median TDI score: 4 (IQR 1–8.5) | NR | NR |
| Altundag (2021) | Anosmia (n = 62) | Clinical history; Four‐item odor identification test in COVID‐19 anosmia patients; Sniffin' Sticks olfactory test for all participants |
Four‐item odor identification test Mean score: 0 Sniffin' Sticks tests Mean‐threshold (t) value: 1.2 ± 0.5; Discrimination (d): 0.9 ± 1; Identification (i): 1.4 ± 2; Total TDI score: 3.6 ± 3.3; Mean TDI score: 3.60 |
Sniffin' Sticks tests Anosmia due to viral infection other than COVID‐19—Mean t value: 1.7 ± 2; d value: 1.5 ± 1.7; i value: 2.3 ± 2.7; Total TDI score: 5.5 ± 5.1; Mean TDI score: 4.54 Healthy controls—Mean t value: 11 ± 1; d value: 12.5 ± 1.5; i value: 13.3 ± 0.7; Total TDI: 35 ± 2.3; Mean TDI score: 38.07 |
NR |
| Yıldız (2021) | Mild hyposmia (n = 13); moderate hyposmia (n = 13); severe hyposmia (n = 9); anosmia (n = 5) | Connecticut Chemosensory Clinical Research Center (CCCRC) olfactory test | Mild hyposmia (n = 13); moderate hyposmia (n = 13); severe hyposmia (n = 9); anosmia (n = 5) | NR | NR |
| Naeini (2020) | Anosmia | NR | NR | NR | NR |
| Niesen (2021) | Hyposmia (n = 5); Anosmia (n = 7) | Identification test included in Sniffin' Sticks battery; qualitative anamnesis and visual analog scale | Sniffin' Sticks identification test: Mean identification test score: 8/16 (± 1.4); Documented hyposmia (scores between 9 and 11): 5 individuals; Documented anosmia (score ≤8): 7 individuals | NR | NR |
NR = not reported; OD = olfactory dysfunction; TDI = threshold discrimination identification.
Data on individual patient outcomes were available for 297 patients (208 with COVID‐19 infection and OD, 10 with COVID‐19 infection and normosmia, and 79 normosmic patients without COVID‐19). In cases where imaging findings from control subjects were not readily available for comparison, abnormalities in olfactory structures were reported based on the interpreting radiologist's clinical expertise. The compiled radiological outcomes from individual cases are summarized in Table IV, and pictorially represented in the Figure 2.
TABLE IV.
Individual Case Compilation of Radiological Outcomes in Olfactory Structures in 297 Patients.
| Individual Patients (n = 297) | ||||||
|---|---|---|---|---|---|---|
| COVID Patients With Olfactory Dysfunction (n = 208) | Controls (n = 89; 10 COVID‐19 Without OD, 79 Healthy) | |||||
| With Abnormalities | Without Abnormalities | With Abnormalities | Without Abnormalities | |||
| Radiological Feature | No. of Patients | Specific Abnormalities | No. of Patients | No. of Patients | Specific Abnormalities | No. of Patients |
| Olfactory bulb volume | 12/20 (60%) |
Severe enlargement with bilateral olfactory bulb edema (n = 1) 41 Smaller right olfactory bulb (n = 1) 40 Olfactory bulb atrophy and/or volume loss (n = 10) 15 , 25 , 33 |
8/20 (40%) 25 , 28 , 30 , 34 , 39 , 44 , 46 | 0/5 (0%) | 5/5 (100%) 30 , 33 | |
| Olfactory bulb morphology | 18/39 (46.2%) |
Olfactory bulb asymmetry (n = 3) 36 Mild irregularity with preserved J‐shape (n = 2) 13 Contour lobulations (n = 5) 13 Rectangular shape (n = 8) 13 |
21/39 (53.8%) 13 , 25 , 36 , 39 | 0/8 (0%) | 8/8 (100%) 25 | |
| Olfactory bulb signal intensity | 42/50 (84%) |
Bilateral olfactory bulb hyperintensity (n = 8) 14 , 29 , 30 Unilateral olfactory bulb hyperintensity (n = 1) 29 Hyperintensity, not stated if unilateral or bilateral (n = 14) 24 , 32 , 41 , 42 Diffusely increased signal (n = 9) 13 Hyperintense foci without halo (n = 11) 13 Hyperintense foci with halo (n = 5) 13 Microhemorrhages (n = 4) 13 |
8/50 (16%) 39 , 44 , 45 , 46 | 15/16 (93.4%) |
Bilateral olfactory bulb hyperintensity (n = 3) (COVID‐19 without OD) 30 Unilateral olfactory bulb hyperintensity (n = 1) (COVID‐19 without OD) 29 Hyperintensity, not stated if unilateral or bilateral (n = 11) (10 healthy, 1 COVID‐19 without OD) 24 , 32 |
1/16 (6.6%) 29 |
| Olfactory cleft swelling | 1/1 (100%) | Mild olfactory cleft edema (n = 1) 41 | ||||
| Olfactory cleft volumes | 0/2 (0%) | 2/2 (100%) 41 , 46 | ||||
| Olfactory cleft opacification | 66/115 (57.4%) |
Bilateral olfactory cleft obstruction (n = 49) 13 , 22 , 23 , 36 Unilateral signs of inflammation or olfactory cleft obstruction (n = 4) 13 , 36 Not stated if unilateral or bilateral olfactory cleft opacification (n = 13) 27 |
49/115 (42.6%) 22 , 23 , 27 , 30 , 36 | 3/67 (4.5%) |
Bilateral olfactory cleft opacification (n = 1) (COVID‐19 without OD) 23 Not stated if unilateral or bilateral olfactory cleft opacification (n = 2) (healthy) 27 |
64/67 (55.5%) 22 , 23 , 27 , 30 |
| Olfactory cleft signal intensity | 0/1 (0%) | 1/1 (100%) 46 | ||||
| Olfactory sulcus depth | 0/1 (0%) | 1/1 (100%) 39 | ||||
| Olfactory sulcus signal intensity | 0/1 (0%) | 1/1 (100%) 39 | ||||
| Olfactory mucosa | 4/58 (6.9%) |
Olfactory mucosa thickening (n = 3) 25 Olfactory mucosa thickening with enhancement of olfactory mucosa compared to nasal mucosa (n = 1) 25 |
44/58 (93.1%) 14 , 25 , 34 | 0/8 (0%) | 8/8 (100%) 25 | |
| Olfactory gyrus | 3/3 (100%) |
Hypometabolism of bilateral olfactory–rectal gyrus (n = 1) 37 Hyperintensity in posterior portion of right rectal gyrus (n = 1) 42 Bilateral cortical hyperintensity predominantly involving the rectus gyrus overlying the olfactory tract and olfactory bulb on MRI 47 |
1/1 (100%) | · Hypometabolism of bilateral olfactory–rectal gyrus (n = 1) (COVID‐19 without OD) 37 | ||
| Olfactory nerve morphology–filia architecture | 10/23 (43.5%) |
Evident clumping of olfactory filia (n = 8) 13 Thinning with scarcity of filia (n = 2) 13 |
13/23 (56.5%) 13 | |||
| Olfactory nerve signal intensity | 1/1 (100%) | Linear hyperintensities in bilateral olfactory nerves (n = 1) 40 | ||||
OD = olfactory dysfunction.
Fig. 2.
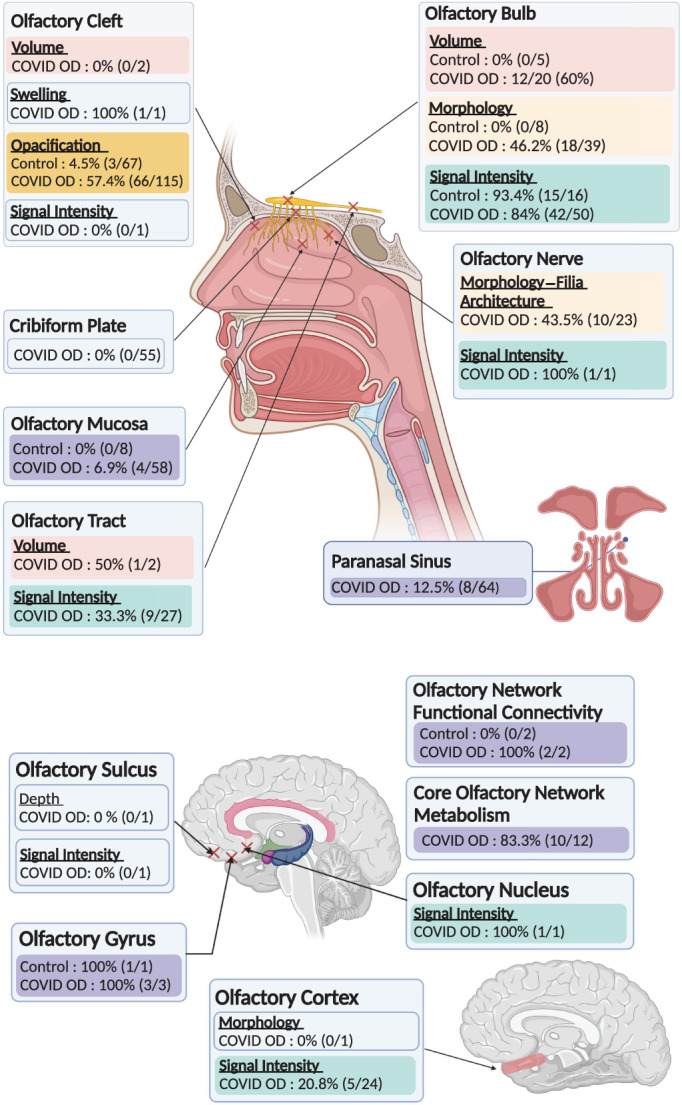
Graphical summary of individual case compilation of radiological outcomes in the olfactory structures. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Olfactory Cleft Opacification
Individual data on olfactory cleft opacification was available in 115 patients with COVID‐19 infection and OD. Of these 115 patients, 66 had olfactory cleft opacification noted on CT and/or MRI (57.4%). MRI demonstrated bilateral and unilateral olfactory cleft opacification in 49 patients 16 , 26 , 27 , 40 and 4 patients, respectively. 16 , 40 In 13 patients imaged using CT, it was not reported if opacification was found in only one or both olfactory clefts. 31 Figure 3 shows T2‐weighted coronal MR image demonstrating complete opacification of bilateral olfactory clefts in a patient with COVID‐19 and OD. 40
Fig. 3.
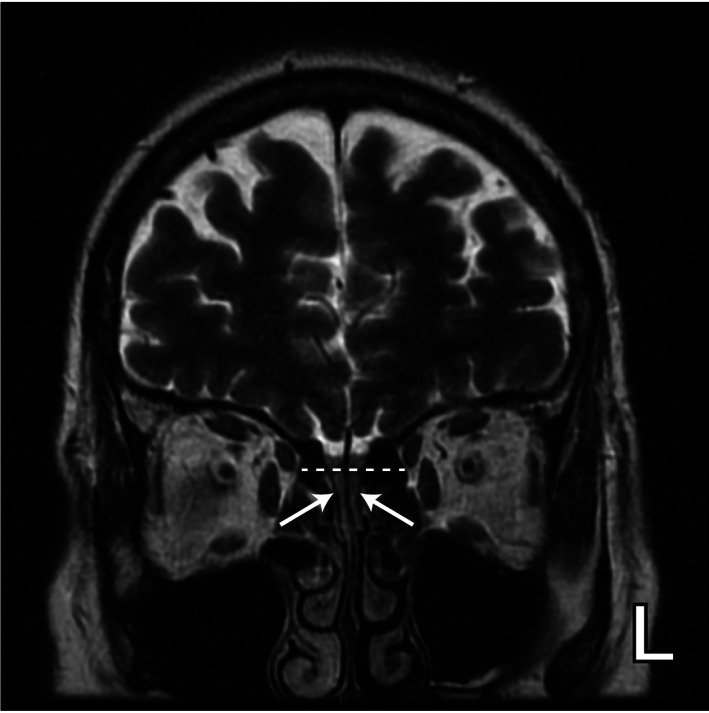
T2‐weighted coronal MR image demonstrating complete opacification of bilateral olfactory clefts (white arrows) in a patient with COVID‐19 and olfactory dysfunction (reproduced with permission from Niesen et al.).
In the control group, 3 out of 67 patients had olfactory cleft opacification (4.5%). One patient with COVID‐19 infection without OD had bilateral olfactory cleft opacification seen on MRI. 27 In the two patients without COVID‐19 infection and OD, it was not stated if opacification affected one or both clefts on CT. 31
Data on olfactory cleft opacification in patients with COVID‐19 infection and OD, and control subjects, was reported in five aggregate studies. 16 , 26 , 27 , 31 , 40 Data from these aggregate studies was pooled in a meta‐analysis, along with individual patient data compiled from case reports and small case series, as demonstrated in Figure 4. The pooled prevalence of olfactory cleft opacification in patients with COVID‐19 infection and OD was 63% (95% CI = 0.38–0.82, I 2 = 73%, N = 115), which was significantly higher than the pooled prevalence of 4% (95% CI = 0.01–0.13, I 2 = 0%, N = 67) in control subjects, which consisted of 7 normosmic COVID‐19 patients, and 60 normosmic non‐COVID‐19 individuals.
Fig. 4.
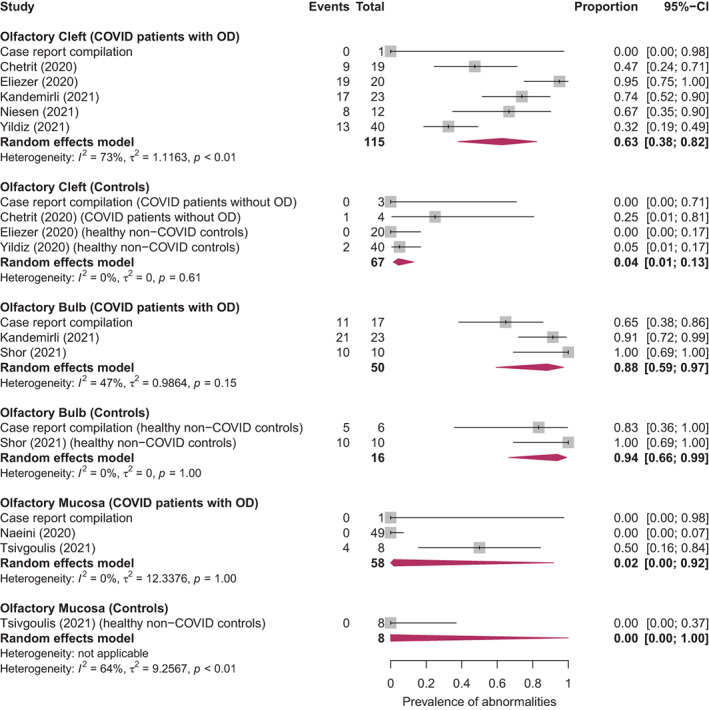
Generalized linear mixed models random‐effects meta‐analysis of the pooled prevalence of olfactory cleft opacification, olfactory bulb signal abnormalities, and olfactory mucosa abnormalities, in patients with COVID‐19‐associated olfactory dysfunction and in controls. Red diamonds are the estimated pooled prevalence for each random‐effects meta‐analysis; gray box sizes reflect the relative weight apportioned to studies in the meta‐analysis. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Other Olfactory Cleft Findings
Olfactory cleft edema was noted in one patient with COVID‐19 infection and OD on MRI. 45 Normal olfactory cleft volumes were detected in two patients with COVID‐19 infection and OD on MRI. 45 , 50
Olfactory Bulb Volumes
Among the 20 patients with COVID‐19 infection and OD, severe enlargement and edema of bilateral olfactory bulbs were detected in 1 patient 7 days after COVID‐19 diagnosis on MRI (5%). 45 Conversely, small olfactory bulbs and/or olfactory bulb atrophy were noted in 11 patients on MRI (55%). Of these 11 patients, imaging was performed on 23 days, 18 28 days, 44 and more than 40 days 29 after the onset of OD in 1, 1, and 7 patients, respectively. Time of imaging in relation to symptom onset was not reported in two patients. 37
Of the three patients with COVID‐19 infection and normal olfactory status in the control group, none had abnormally sized olfactory bulbs seen on MRI (0%). 34
Olfactory Bulb Morphology
Abnormal olfactory bulb morphology was detected in 18 out of 39 patients with COVID‐19 infection and OD (46.2%). Of these 18 patients, MRI revealed olfactory bulb asymmetry in 3 patients. 40 In another study in which oval or inverted J‐shape was considered the normal olfactory bulb morphology, two, five, and eight patients had mild irregularity of the olfactory bulb with preserved J‐shape, contour lobulations, and rectangular‐shaped olfactory bulbs on MRI, respectively. 16
The eight patients in the control group without COVID‐19 infection and OD did not have morphological abnormalities of the olfactory bulb (0%). 29
Olfactory Bulb Signal Intensity
Olfactory bulb signal hyperintensity was detected in 42 out of 50 patients with COVID‐19 infection and OD on MRI (84%). Of these 42 patients, 8 patients had bilateral olfactory bulb hyperintensity, 17 , 33 , 34 1 had unilateral olfactory bulb intensity, 33 while it was not specifically reported if hyperintensity was unilateral or bilateral in 14 patients. 28 , 36 , 45 , 46 In addition, 9, 11, 5, and 4 patients had diffusely increased signal, hyperintense foci without halo, hyperintense foci with halo, and microhemorrhages in the olfactory bulb, respectively. 16 MRI sequences employed consisted of combinations of T1‐weighted, T2‐weighted, STIR, FLAIR, diffusion‐weighted, and other additional conventional whole‐brain sequences, with contrast used in two studies. 33 , 36 , 51
Conversely, olfactory bulb hyperintensity was noted in 15 out of 16 controls on MRI (93.4%). Five of them had COVID‐19 infection without OD—three had bilateral hyperintensity, 34 one had unilateral hyperintensity, 33 while the location of hyperintensity was not described in the remaining patient. 36 The other 10 patients with olfactory bulb hyperintensity were healthy controls, without data on the location of hyperintensity. 28
Data on olfactory bulb signal abnormalities in patients with COVID‐19 infection and OD, as well as control subjects, was reported in two aggregate studies. 16 , 28 Data from these aggregate studies was pooled in a meta‐analysis, along with individual patient data compiled from case reports and small case series, as demonstrated in Figure 4. The pooled prevalence of olfactory bulb signal abnormalities in patients with COVID‐19 infection and OD was 88% (95% CI = 0.59–0.97, I 2 = 47%, N = 50), while the pooled prevalence in control subjects was 94% (95% CI = 0.66–0.99, I 2 = 0%, N = 16), all of whom were normosmic non‐COVID‐19 individuals. Hence, olfactory bulb signal abnormalities appear to be equally represented in both cases and controls.
Olfactory Mucosa
Four out of 58 patients with COVID‐19 infection and OD had radiological abnormalities of the olfactory mucosa (6.9%). Four patients had thickening and edema of the olfactory mucosa seen on MRI of the brain and olfactory bulb, one of whom also had enhancement of the olfactory mucosa relative to the nasal mucosa on postcontrast media infusion. 29
None of the eight patients in the control group who did not have COVID‐19 infection and OD had radiological abnormalities of the olfactory mucosa. 29
Data on olfactory mucosa abnormalities in patients with COVID‐19 infection and OD, and control subjects, was reported in two aggregate studies. 29 , 38 Data from these aggregate studies was pooled in a meta‐analysis, along with individual patient data compiled from case reports and small case series, as demonstrated in Figure 4. The pooled prevalence of olfactory mucosa abnormalities in patients with COVID‐19 infection and OD was 2% (95% CI = 0.00–0.92, I 2 = 0%, N = 58), while the pooled prevalence in control subjects was 0% (95% CI = 0.00–1.00, I 2 = 64%, N = 8), all of whom were normosmic non‐COVID‐19 individuals.
Olfactory Tract Volumes
One of two patients with COVID‐19 infection and OD had olfactory tract edema detected on MRI (50%). In this patient, the olfactory tract was swollen in the central portion and edema was more prominent in the right olfactory tract. 17
Olfactory Tract Signal Intensity
Olfactory tract hyperintensity was noted in 9 out of 27 patients with COVID‐19 infection and OD on MRI (33.3%). Two had bilateral olfactory tract hyperintensity seen with FLAIR, T2 fast recovery fast spin‐echo, and post‐gadolinium T1 sequences, 17 , 51 while seven had diffusely increased olfactory tract signal visualized with T2‐weighted and conventional whole brain sequences. 16
Olfactory Gyrus
Radiological abnormalities of the olfactory gyrus were noted in three out of three patients with COVID‐19 infection and OD (100%). One patient had metabolic changes in the olfactory gyrus in the form of hypometabolism of the bilateral olfactory–rectus gyri detected on [18F]‐fluorodeoxyglucose (FDG) PET, 41 while MRI revealed hyperintensity in the posterior portion of the right rectal gyrus in another patient. 46 The third patient in this group had bilateral cortical hyperintensity predominantly involving the rectus gyrus overlying the olfactory tract and olfactory bulb on MRI. 51
Hypometabolism of bilateral olfactory–rectus gyri was noted in one patient with COVID‐19 infection without OD on 18F‐FDG PET. 41
Olfactory Nerve Morphology and Filia Architecture
Ten out of 23 of the patients with COVID‐19 infection and OD demonstrated disruptions to the olfactory nerve morphology or filia architecture (43.5%). Dedicated olfactory nerve MRI showed obvious clumping of the olfactory filia and thinning with scarcity of filia in eight and two patients, respectively. 16
Cribriform Plate
Out of 55 patients with COVID‐19 infection and OD, none demonstrated any cribriform plate abnormalities (0%). The cribriform plate was visualized using paranasal sinus CT scans in 49 patients, 38 and MRI in 6 patients. 32 , 39
Olfactory Cortex Signal Intensity
Olfactory cortex hyperintensity was noted in 5 out of 24 patients with COVID‐19 infection and OD on MRI (20.8%), 16 while no abnormal signal intensities were demonstrated in the other 19 patients. 16 , 48
Core Olfactory Network Metabolism
Using 18F‐FDG PET imaging, 10 out of 12 patients with COVID‐19 infection and OD were identified to have abnormal metabolism in the core olfactory network (83.3%). Of these patients, four had hypometabolism and six had hypermetabolism in the nodes of the core olfactory network, which are structures involved in odor processing. 40
Paranasal Sinus
Paranasal sinus abnormalities were noted in 8 out of 64 patients with COVID‐19 infection and OD (12.5%). On MRI, two and four patients demonstrated minimal mucosal thickening in the ethmoid sinuses, 43 and signs of inflammation or partial obliteration in the ethmoid, sphenoid, and/or maxillary sinuses, respectively. 40 In two patients, discrete signs of sinus inflammation were similarly noted on MRI without specification of the involved sinuses. 40
DISCUSSION
OD can be broadly classified into conductive and sensorineural types. 52 The conductive subtype is characterized by physical obstruction of airflow to the olfactory mucosa, and is commonly seen in nasal and paranasal sinus disorders, with reasonably good prognosis following treatment. The sensorineural subtype arises due to disruption of the olfactory‐neural signaling pathways, and has been attributed to upper respiratory tract infections, neurodegenerative disorders, trauma, and toxins, with significantly poorer prognosis. 53 Hummel et al. has also proposed a third subtype—central dysfunction, which arises due to damage to the olfactory processing pathways of the central nervous system. 54
In this systematic review and meta‐analysis, we demonstrated that olfactory cleft opacification may play a significant role in the pathogenesis of COVID‐19‐associated OD. The pooled prevalence of olfactory cleft opacification was nearly 16‐fold higher in patients with COVID‐19 infection and OD (63%), compared to controls (4%). As integral olfactory structures, the olfactory clefts provide a crucial channel for airborne odorant molecules to reach the olfactory mucosa. 55 The sensorial olfactory neurons of the olfactory mucosa then pass through the cribriform lamina and form the olfactory bulb, which connects to the brain via the olfactory tract. 56
Conversely, our current findings do not support the involvement of the olfactory bulb and olfactory mucosa in the pathogenesis of COVID‐19‐associated OD. Normal adult‐type olfactory bulbs have been described in literature to demonstrating intermediate, uniform T2 signal intensity from the center to the periphery on MRI. 57 , 58 Our meta‐analysis demonstrated that the pooled prevalence of olfactory bulb signal abnormalities was largely similar in patients with COVID‐19 infection and OD (88%), and control subjects (94%). The high proportion of abnormal findings in both cases and controls could be attributed to the non‐specific nature of olfactory bulb signal abnormalities, which may arise due to degeneration or microbleeding, 33 and the tendency for signal artifacts in the olfactory bulb region. 59 Similarly, the pooled prevalence of olfactory mucosa abnormalities was low in both patients with COVID‐19 infection and OD (2%), and control subjects (0%), with overlapping confidence intervals.
At present, conductive mechanisms of olfactory loss in COVID‐19‐associated OD have largely been dismissed in favor of sensorineural mechanisms, 60 , 61 given the absence of significant sinonasal symptoms like nasal obstruction and mucosal congestion in COVID‐19 infection. 60 , 62 However, our findings suggest that conductive mechanisms may play a crucial role in mediating smell loss in 63% of patients. Obstruction and edema of the olfactory cleft can impede airflow and block odor molecules from reaching the intact olfactory epithelium, resulting in conductive smell loss. 63 Moreover, the excellent prognosis of COVID‐19‐associated OD, with complete recovery in nearly 80% of patients in the first 2 months following resolution of acute inflammation, 64 appears more consistent with initial conductive olfactory loss. Nonetheless, involvement of the olfactory bulb and mucosa cannot be excluded, as current imaging modalities may be inadequate in detecting true viral‐mediated damage to these structures. In the small minority of patients with persistent anosmia, delayed recovery may be secondary to severe olfactory epithelium inflammation causing scarring and impairment of regeneration, 65 or damage to the olfactory receptor neurons and stem cell neurons. 63 In post‐viral anosmia due to non‐COVID‐19 infections, the small subset of patients who experience permanent olfactory loss was similarly found to have virus‐induced sensory neuronal damage. 66
Currently, the clinical significance of olfactory cleft opacification in COVID‐19‐associated OD is not fully known. In other rhinologic conditions like chronic rhinosinusitis, olfactory cleft opacification may carry prognostic significance, 12 with the ability to predict olfactory function scores, 12 and outcomes following functional endoscopic sinus surgery. 67 It would be clinically valuable to explore prognostic studies investigating radiological findings in relation to anosmia recovery in COVID‐19 infection.
Moreover, few cribriform plate (0%) and paranasal sinus abnormalities (12.5%) were detected in patients with COVID‐19‐associated OD, suggesting these structures are unlikely involved in the underlying pathogenesis. The cribriform plate supports the olfactory bulb, and is perforated by olfactory foramina for the passage of the olfactory nerves and anterior ethmoidal nerves to the roof of the nasal cavity to convey smell to the central nervous system. 68 Radiological abnormalities of the cribriform plate have largely been reported in cases of anterior skull trauma. 69 Conversely, radiological abnormalities of the paranasal sinuses are frequently observed in inflammatory sinonasal disorders. 70
Apart from COVID‐19 infection, OD may also be a feature of other viral infections. 71 Post‐viral anosmia arises due to underlying mucosal congestion and nasal obstruction causing conductive olfactory loss. 72 In one included study, 30 38 control subjects had anosmia secondary to non‐COVID‐19 viral infections. While olfactory cleft widths, volumes, and T2 signal intensities were significantly increased in COVID‐19 and non‐COVID‐19 viral anosmia groups compared to healthy controls, there were no significant differences in these parameters between both anosmia groups. Therefore, it is possible that COVID‐19 and other viral infections may share similarities in their pattern of involvement of olfactory structures.
Strengths and Limitations
To the best of our knowledge, this is the first meta‐analysis pooling and analyzing radiological changes to olfactory structures in patients with COVID‐19 infection and OD. A wide variety of radiological outcomes in specific olfactory structures was investigated.
Nonetheless, this study should be interpreted in the context of known and potential limitations. First, data from case reports and small case series were included. These studies are inherently subjected to higher risk of bias than consecutive case series, cohort studies, and case–control studies. However, given the resource constraints of the pandemic, and difficulty in procuring MRI findings, such a review decision was necessary. As seen in Figure 4, the compilation of case reports yielded a prevalence remarkably similar to that of aggregate studies and pooled effect, and exclusion of case reports did not substantially change the pooled effect.
Second, several radiological outcomes lack data pertaining to control subjects, including abnormalities in the paranasal sinus, olfactory nerve, olfactory tract, and olfactory cortex. This has limited our ability to draw robust conclusions about their significance. Greater access to studies reporting radiological abnormalities in these structures in wider populations may elucidate their clinical significance in COVID‐19‐associated OD.
In addition, only 10 out of 89 control subjects in this study had COVID‐19 infection and normosmia, of which 7 demonstrated structural and/or functional abnormalities (70%). Given the small number of patients in this group, the significance of these findings is uncertain. Normosmic COVID‐19 individuals may also demonstrate sub‐clinical radiological abnormalities, since the SARS‐CoV‐2 virus has been postulated to infect individuals via a similar pathway with colonization and inflammation of the nasal respiratory and olfactory epithelium. 73 Nonetheless, a radiological study (N = 55) found that few normosmic COVID‐19 showed significant nasopharyngeal thickness (7%) or olfactory cleft opacification (7%) on CT or MRI. 74 With substantial heterogeneity in current studies, extensive analysis of olfactory imaging findings in a significant population of COVID‐19 normosmic patients is required.
Furthermore, there was significant between‐study heterogeneity in terms of imaging protocols. Among the 12 aggregate studies included, dedicated olfactory system MRI was performed only in Kandemirli, 16 and Altundag. 30 Instead, most included studies either added one sequence for the olfactory region or utilized whole brain MRI scans. As such, subtle abnormalities in olfactory structures could have been undetected. The olfactory nerve, being small, is also best visualized on dedicated skull base MRI scans. 18 In addition, most studies lacked specific control or pre‐contrast, and blood‐specific sequences. Study participants also largely did not have baseline scans taken before the onset of COVID‐19 infection to account for pre‐existing radiological abnormalities. Therefore, this poses a challenge in determining if olfactory imaging abnormalities can be attributed solely to COVID‐19 infection and OD.
Future studies should investigate radiological changes to the olfactory structures in a longitudinal fashion. By following patients up over a protracted duration, improvement or progression of imaging findings can be tracked, with correlation to patients' clinical symptoms. Changes in radiological findings in response to targeted treatment for OD may also be useful to monitor, and may inform the underlying mechanisms of COVID‐19‐associated OD. In addition, other long‐term clinical outcomes following COVID‐19 infection, including post‐infectious cognitive outcomes, 9 can also be explored. Lastly, correlation of our findings with histopathological studies may potentially reveal greater mechanistic data. However, large cohort studies investigating histopathological findings are largely unfeasible, requiring either invasive endoscopic biopsy, or post‐mortem autopsy in deceased patients who had COVID‐19‐associated OD.
CONCLUSION
In this systematic review and meta‐analysis, we investigated a wide range of radiological abnormalities of the olfactory structures in COVID‐19‐associated OD. Olfactory cleft opacification is a key radiological marker of COVID‐19‐associated OD, while other findings like olfactory bulb signal abnormalities, and olfactory mucosa abnormalities, appear less related. This has mechanistic implications and suggests that conductive mechanisms of olfactory loss may have an important role in the pathogenesis of COVID‐19‐associated OD. Such imaging findings may also guide prognostication and treatment of OD. Inclusion of studies with larger sample sizes, robust study designs, data on normosmic COVID‐19 controls, and consistent imaging techniques specific to the olfactory system will allow more reliable conclusions to be drawn. Nevertheless, given the resource constraints, our findings represent the best available current evidence in the context of the pandemic.
Supporting information
Appendix S1: Supporting information.
ACKNOWLEDGMENTS
Graphical summary (Figure 2) was created with BioRender.com.
b.k.j.t. gratefully acknowledges the SingHealth Medical Student Talent Development Award (SMSTDA)–Project. All other authors have no funding sources to declare.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Pokharel A. Olfactory dysfunction: a clinical marker of COVID‐19. J Nepal Med Assoc 2021;59(233):88–93. 10.31729/jnma.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pang KW, Chee J, Subramaniam S, Ng CL. Frequency and clinical utility of olfactory dysfunction in COVID‐19: a systematic review and meta‐analysis. Curr Allergy Asthma Rep 2020;20(12):76. 10.1007/s11882-020-00972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilinska K, Butowt R. Anosmia in COVID‐19: a bumpy road to establishing a cellular mechanism. ACS Chem Nerosci 2020;11(15):2152–2155. 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID‐19: diagnosis and management. JAMA 2020;323(24):2512–2514. 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11(7):995–998. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 6. De Santis G. SARS‐CoV‐2: a new virus but a familiar inflammation brain pattern. Brain Behav Immun 2020;87:95–96. 10.1016/j.bbi.2020.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82(15):7264–7275. 10.1128/jvi.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzo C, Serra‐Mestres J, Isetta M, Castagna A. Could COVID‐19 anosmia and olfactory dysfunction trigger an increased risk of future dementia in patients with ApoE4? Med Hypotheses 2021;147:110479. 10.1016/j.mehy.2020.110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pirker‐Kees A, Platho‐Elwischger K, Hafner S, Redlich K, Baumgartner C. Hyposmia is associated with reduced cognitive function in COVID‐19: first preliminary results. Dement Geriatr Cogn Disord 2021;50(1):68–73. 10.1159/000515575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rombaux P, Duprez T, Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 2009;47(1):3–9. [PubMed] [Google Scholar]
- 11. Duprez TP, Rombaux P. Imaging the olfactory tract (cranial nerve #1). Eur J Radiol 2010;74(2):288–298. 10.1016/j.ejrad.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 12. Chang H, Lee HJ, Mo JH, Lee CH, Kim JW. Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg 2009;135(10):988–992. 10.1001/archoto.2009.140. [DOI] [PubMed] [Google Scholar]
- 13. Jobin B, Boller B, Frasnelli J. Volumetry of olfactory structures in mild cognitive impairment and Alzheimer's disease: a systematic review and a meta‐analysis. Brain Sci 2021;11(8):6–13. 10.3390/brainsci11081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tekcan Sanli DE, Altundag A, Yıldırım D, Kandemirli SG, Sanli AN. Comparison of olfactory cleft width and volumes in patients with COVID‐19 anosmia and COVID‐19 cases without anosmia. ORL J Otorhinolaryngol Relat Spec Sep 2021;21:1–9. 10.1159/000518672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altunisik E, Baykan AH, Sahin S, Aydin E, Erturk SM. Quantitative analysis of the olfactory system in COVID‐19: an MR imaging study. AJNR Am J Neuroradiol 2021;42:2207–2214. 10.3174/ajnr.A7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID‐19 anosmia. Acad Radiol 2021;28(1):28–35. 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melegari G, Rivi V, Zelent G, et al. Mild to severe neurological manifestations of COVID‐19: cases reports. Int J Environ Res Public Health 2021;18(7):3673. 10.3390/ijerph18073673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiu A, Fischbein N, Wintermark M, Zaharchuk G, Yun PT, Zeineh M. COVID‐19‐induced anosmia associated with olfactory bulb atrophy. Neuroradiology 2021;63(1):147–148. 10.1007/s00234-020-02554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. University of Ottawa; 2014. [Google Scholar]
- 21. Higgins JPT, Altman DG, Sterne JAC . Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
- 22. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta‐analyses: advantages and limitations of the Newcastle Ottawa scale. World J Meta‐Anal 2017;5:80. 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 23. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta‐analysis of RCTs using standard and generalised Qstatistics. BMC Med Res Methodol 2011;11(1):41. 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, Griffith L, Oremus M, Raina P, Ismaila A, Santaguida P, Lau J, Trikalinos TA. AHRQ Methods for Effective Health Care Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. Methods Guide for Effectiveness and Comparative Effectiveness Reviews: Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 25. Güney B, Bacaksızlar Sarı F, Özdemir MY, Çullu N, Doğan E, Togan T. Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID‐19 infection. Acta Otolaryngol 2021;141(8):786–790. 10.1080/00016489.2021.1946138. [DOI] [PubMed] [Google Scholar]
- 26. Eliezer M, Hamel AL, Houdart E, et al. Loss of smell in patients with COVID‐19: MRI data reveal a transient edema of the olfactory clefts. Neurology 2020;95(23):e3145–e3152. 10.1212/wnl.0000000000010806. [DOI] [PubMed] [Google Scholar]
- 27. Chetrit A, Lechien JR, Ammar A, et al. Magnetic resonance imaging of COVID‐19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect 2020;81(5):816–846. 10.1016/j.jinf.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shor N, Chougar L, Pyatigorskaya N. MR imaging of the olfactory bulbs in patients with COVID‐19 and anosmia: how to avoid misinterpretation. AJNR Am J Neuroradiol 2021;42(3):E10–e11. 10.3174/ajnr.A6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsivgoulis G, Fragkou PC, Lachanis S, et al. Olfactory bulb and mucosa abnormalities in persistent COVID‐19‐induced anosmia: a magnetic resonance imaging study. Eur J Neurol 2021;28(1):e6–e8. 10.1111/ene.14537. [DOI] [PubMed] [Google Scholar]
- 30. Altundag A, Yıldırım D, Tekcan Sanli DE, et al. Olfactory cleft measurements and COVID‐19‐related anosmia. Otolaryngol Head Neck Surg 2021;164(6):1337–1344. 10.1177/0194599820965920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yıldız E, Balcı A, Selendili O, Kuzu S. Olfactory cleft opacification in COVID‐19 related smell loss: CT findings and correlation with objective testing. Ear Nose Throat J 2021;1–7. 10.1177/01455613211011285. [DOI] [PubMed] [Google Scholar]
- 32. Brookes NRG, Fairley JW, Brookes GB. Acute olfactory dysfunction‐a primary presentation of COVID‐19 infection. Ear Nose Throat J 2020;99(9):94–98. 10.1177/0145561320940119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aragão M, Leal MC, Cartaxo Filho OQ, Fonseca TM, Valença MM. Anosmia in COVID‐19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol 2020;41(9):1703–1706. 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin E, Lantos JE, Strauss SB, et al. Brain imaging of patients with COVID‐19: findings at an academic institution during the height of the outbreak in New York City. Am J Neuroradiol 2020;41:2001–2008. 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lechien JR, Michel J, Radulesco T, et al. Clinical and radiological evaluations of COVID‐19 patients with anosmia: preliminary report. Laryngoscope 2020;130(11):2526–2531. 10.1002/lary.28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aragao M, Leal MC, Andrade PHP, et al. Clinical and radiological profiles of COVID‐19 patients with neurological symptomatology: a comparative study. Viruses 2021;13(5):1–13. 10.3390/v13050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung TW, Zhang H, Wong FK, et al. Neurosensory rehabilitation and olfactory network recovery in Covid‐19‐related olfactory dysfunction. Brain Sci 2021;11(6):1–4. 10.3390/brainsci11060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naeini AS, Karimi‐Galougahi M, Raad N, et al. Paranasal sinuses computed tomography findings in anosmia of COVID‐19. Am J Otolaryngol 2020;41(6):102636. 10.1016/j.amjoto.2020.102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schönegger CM, Gietl S, Heinzle B, Freudenschuss K, Walder G. Smell and taste disorders in COVID‐19 patients: objective testing and magnetic resonance imaging in five cases. SN Compr Clin Med 2020;24:1–5. 10.1007/s42399-020-00606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niesen M, Trotta N, Noel A, et al. Structural and metabolic brain abnormalities in COVID‐19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging 2021;48(6):1890–1901. 10.1007/s00259-020-05154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guedj E, Million M, Dudouet P, et al. (18)F‐FDG brain PET hypometabolism in post‐SARS‐CoV‐2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging 2021;48(2):592–595. 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karimi‐Galougahi M, Yousefi‐Koma A, Bakhshayeshkaram M, Raad N, Haseli S. (18)FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID‐19. Acad Radiol 2020;27(7):1042–1043. 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ismail II, Gad KA. Absent blood oxygen level‐dependent functional magnetic resonance imaging activation of the orbitofrontal cortex in a patient with persistent cacosmia and cacogeusia after COVID‐19 infection. JAMA Neurol 2021;78(5):609–610. 10.1001/jamaneurol.2021.0009. [DOI] [PubMed] [Google Scholar]
- 44. Li CW, Syue LS, Tsai YS, et al. Anosmia and olfactory tract neuropathy in a case of COVID‐19. J Microbiol Immunol Infect 2021;54(1):93–96. 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID‐19‐related anosmia. Neurology 2020;95(5):224–225. 10.1212/wnl.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 46. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID‐19) and anosmia. JAMA Neurol 2020;77(8):1028–1029. 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 47. Corrêa DG, Hygino da Cruz LC Jr, FCR L, et al. Magnetic resonance imaging features of COVID‐19‐related cranial nerve lesions. J Neurovirol 2021;27(1):171–177. 10.1007/s13365-020-00934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yousefi‐Koma A, Haseli S, Bakhshayeshkaram M, Raad N, Karimi‐Galougahi M. Multimodality imaging with PET/CT and MRI reveals hypometabolism in tertiary olfactory cortex in parosmia of COVID‐19. Acad Radiol 2021;28(5):749–751. 10.1016/j.acra.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, Naeini AS, Haseli S. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol 2020;27(6):892–893. 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vaira LA, Hopkins C, Sandison A, et al. Olfactory epithelium histopathological findings in long‐term coronavirus disease 2019 related anosmia. J Laryngol Otol 2020;134(12):1123–1127. 10.1017/s0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Casez O, Willaume G, Grand S, et al. Teaching NeuroImages: SARS‐CoV‐2‐related encephalitis: MRI pattern of olfactory tract involvement. Neurology 2021;96(4):e645–e646. 10.1212/wnl.0000000000011150. [DOI] [PubMed] [Google Scholar]
- 52. Wrobel BB, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin North Am 2004;12(4):459–468, vii. 10.1016/j.fsc.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cho SH. Clinical diagnosis and treatment of olfactory dysfunction. Hanyang Med Rev 2014;34:107. 10.7599/hmr.2014.34.3.107. [DOI] [Google Scholar]
- 54. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl 2017;54(26):1–30. 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 55. Trotier D, Bensimon JL, Herman P, Tran Ba Huy P, Døving KB, Eloit C. Inflammatory obstruction of the olfactory clefts and olfactory loss in humans: a new syndrome? Chem Senses 2007;32(3):285–292. 10.1093/chemse/bjl057. [DOI] [PubMed] [Google Scholar]
- 56. Jafek BW, Murrow B, Michaels R, Restrepo D, Linschoten M. Biopsies of human olfactory epithelium. Chem Senses 2002;27(7):623–628. 10.1093/chemse/27.7.623. [DOI] [PubMed] [Google Scholar]
- 57. Schneider JF, Floemer F. Maturation of the olfactory bulbs: MR imaging findings. Am J Neuroradiol 2009;30(6):1149–1152. 10.3174/ajnr.A1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suzuki M, Takashima T, Kadoya M, Takahashi S, Miyayama S, Taira S. MR imaging of olfactory bulbs and tracts. Am J Neuroradiol 1989;10(5):955–957. [PMC free article] [PubMed] [Google Scholar]
- 59. Tsutsumi S, Ono H, Yasumoto Y. Visualization of the olfactory nerve using constructive interference in steady state magnetic resonance imaging. Surg Radiol Anat 2017;39(3):315–321. 10.1007/s00276-016-1731-9. [DOI] [PubMed] [Google Scholar]
- 60. Butowt R, von Bartheld CS. Anosmia in COVID‐19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist 2020;27:582–603. 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liang F, Wang DY. COVID‐19 anosmia: high prevalence, plural neuropathogenic mechanisms, and scarce neurotropism of SARS‐CoV‐2? Viruses 2021;13(11):2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jalessi M, Barati M, Rohani M, et al. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID‐19. Neurol Sci 2020;41(9):2331–2338. 10.1007/s10072-020-04590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Najafloo R, Majidi J, Asghari A, et al. Mechanism of anosmia caused by symptoms of COVID‐19 and emerging treatments. ACS Chem Nerosci 2021;12(20):3795–3805. 10.1021/acschemneuro.1c00477. [DOI] [PubMed] [Google Scholar]
- 64. Lechien JR, Journe F, Hans S, et al. Severity of anosmia as an early symptom of COVID‐19 infection may predict lasting loss of smell. Front Med 2020;7(716):1–5. 10.3389/fmed.2020.582802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tian J, Pinto JM, Xin Y, et al. Dexamethasone affects mouse olfactory mucosa gene expression and attenuates genes related to neurite outgrowth. Int Forum Allergy Rhinol 2015;5(10):907–918. 10.1002/alr.21586. [DOI] [PubMed] [Google Scholar]
- 66. Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am 2004;37(6):1159–1166. 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 67. Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. Am J Rhinol Allergy 2011;25(2):e90–e94. 10.2500/ajra.2011.25.3617. [DOI] [PubMed] [Google Scholar]
- 68. Barral JP, Croibier A. Manual Therapy for the Cranial Nerves. Chap 4: Cranial nerves as they emerge from the skull. Churchill Livingstone; 2009:19–23. [Google Scholar]
- 69. Gomez JPS. Cribiform Plate Fractures: StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 70. Eggesbø HB. Radiological imaging of inflammatory lesions in the nasal cavity and paranasal sinuses. Eur Radiol 2006;16(4):872–888. 10.1007/s00330-005-0068-2. [DOI] [PubMed] [Google Scholar]
- 71. Suzuki M, Saito K, Min WP, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007;117(2):272–277. 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Welge‐Lüssen A, Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol 2006;63:125–132. 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 73. Han AY, Mukdad L, Long JL, Lopez IA. Anosmia in COVID‐19: mechanisms and significance. Chem Senses 2020;45:423–428. 10.1093/chemse/bjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moonis G, Mitchell R, Szeto B, Lalwani AK. Radiologic assessment of the sinonasal tract, nasopharynx and mastoid cavity in patients with SARS‐Cov‐2 infection presenting with acute neurological symptoms. Ann Otol Rhinol Laryngol 2021;130(11):1228–1235. 10.1177/0003489421995070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.


