Abstract

The synthesis of four derivatives and the single-crystal X-ray structures of six 9-trifluoromethylxanthenediols (TFXdiols) I–VI are analyzed in this work. These compounds were obtained through superacid-catalyzed condensation of dihydroxybenzenes with 1,1,1-trifluoroacetone or 2,2,2-trifluoroacetophenone. The title molecules have a convex molecular structure due to their three fused rings of the xanthene moiety. We have found that, similar to resorcinol, the configuration of the hydroxyl groups is of great relevance for the crystal packing favoring either interactions above and below their molecular plane or lateral interactions that create layers. Considering that reports of TFXdiols are very scarce, our findings contribute to a better understanding of the molecular conformation and intermolecular interactions in their crystal structures. A similar analysis was extended to a fortuitous cocrystal obtained between 9-trifluoromethyl-9-(4′-fluorophenyl)-xanthenediol and 1,4-dihydroxybenzene, showing that these structures might be used to obtain cocrystals in the future.
Introduction
Xanthene-containing molecules have been widely studied due to their numerous applications that take advantage of their biological and pharmacological properties.1−7 They have also attracted interest due to their interesting photophysical properties, as some derivatives can be used as pigments, dyes, and biological sensors.8−14 In addition, xanthene fragments have been used as an essential constituent in polymers chains.15−20 Xanthene fragments are critical components for constructing polymer layered p-electron systems,16−18 as well as polymers for gas separations or sorption, and membranes.19,21,22 Among those, fluorine-containing xanthenes can be used to prepare high-performance heteroaromatic polymers, such as polyimides,23 polyarylethers, and polybenzoxazoles.24 Particularly, the introduction of −CF3 groups in the structure has been one of the most widely used strategies to obtain solution-processable polymers, a highly desired property that enables attractive applications.25,26
Some of us have reported the preparation of soluble polymers based on the condensation of biphenol and 2,2,2-trifluoroacetophenone in superacid conditions.27 In addition, we also employed the symmetric xanthenediol 3,6-dihydroxy-9-trifluoromethyl-9-phenylxanthene (III) to obtain a highly soluble and fully aromatic ladder polymer with excellent gas permselectivity performance.28 By extending the developed approach, new polymers could be obtained using other xanthenediol derivatives.
The xanthene moiety is frequently encountered in the literature (Figure 1a). A recent search in the Cambridge Crystallographic Data Centre,29 performed using ConQuest 2021.1.0 software,30 indicates that approximately 2350 related structures have been deposited, including 9-xanthones (Figure 1b), xanthene dyes (Figure 1c), or xantphos-related structures (Figure 1d). A xanthenediol structure can also be found in the recent work from Niu and collaborators (HORMUZ, Figure 1e).31 Nevertheless, to this date, only one TFXdiol structure has been reported in detail so far (Figure 1f). The reported 3,6-dimethyl-9-phenyl-9-trifluoromethyl-9H-xanthene-2,7-diol was used as an intermediate in the obtaining quinone-hydroquinone hybrid structures in stereodynamic redox reactions.32 Furthermore, only a handful of studies focused on the structure of xanthenes derivatives have been reported, for example, the 9-substituted-9-xanthenol clathrates studied by Taljaard and co-workers,33,34 the 1,8-dioxooctahydroxanthenes analyzed by da Silva and collaborators,35 the 9-substituted-9-phenylxanthenes reported by Kavala and others,36 or mechanochemical cocrystallization of fluorescein by Bučar and others.37
Figure 1.
(a) Xanthene motif (2357 entries in the CCDC). (b) 9-Xanthone motif (264 entries in the CCDC). (c) Xanthene dye skeleton motif (X = O, N, R = −OH, −N(Et)2, 411 entries in the CCDC). (d) Xantphos ligand (423 entries in the CCDC). (e) Related xanthenediol derivative (CCDC code: HORMUZ). (f) 9-Trifluoromethyxanthenediol (TFXdiol) skeleton (1 entry in the CCDC).
Considering the above, in this work, we report the synthesis and structural analysis of four 9-trifluoromethyldihydroxyxanthenediols (TFXdiols). The title compounds were synthesized through condensation catalyzed by trifluoromethanesulfonic acid (TFSA) between resorcinol or hydroquinone and trifluoromethyl ketones.38 Gratifyingly, the resulting compounds show a high tendency to crystallize. It is considered that the study of these X-ray structures would help to establish the most common solid-state conformations and to determine the governing intermolecular interactions (Figure 2a). To facilitate the description of TFXdiols structures, in this work a common numbering system was used for all structures (Figure 2b, Figure S13).
Figure 2.

(a) Molecules and diol conformations in the asymmetric units of compounds I–VI. When two molecules per asymmetric unit are found, labels A and B are employed. Compounds III and V have been published before and were included here only to provide a broader perspective of the packing motifs. (b) Numbering system of this work employed to consistently describe the observed intermolecular interactions.
Results and Discussion
Synthesis and Characterization of Compounds 1–5
Compounds 1–5 were obtained through the reaction of 1,1,1-trifluoroacetone or 2,2,2-trifluoroacetophenone with the appropriate dihydroxybenzene derivative (either hydroquinone, resorcinol, or 2-methylresorcinol) and TFSA in dichloromethane (DCM) at 10 °C (Scheme 1, left). Good yields of each molecule were obtained, and their complete characterization was carried out with diverse analytical techniques as 1H, 13C, and 19F NMR, IR spectroscopy, and mass spectrometry (see Supporting Information). Single crystals suitable for X-ray diffraction were obtained through slow evaporation of saturated solutions of the compounds in DCM. The most relevant crystallographic parameters are compiled in Table 1.
Scheme 1. Left: Synthesis of 9-Trifluoromethylxanthenediols (TFXdiols). Right: Chemical Structures I–VI Analyzed in This Work.
The structures of compounds II and V have been reported before28,32 and are included here for a better description of TFXdiols structures.
Table 1. Selected Crystallographic Data of Crystals I, II, IV, and VI (CCDC Deposit Number).
| I (2122963) | III (2122965) | IV (2122966) | VI (2122964) | |
|---|---|---|---|---|
| formula | 3(C17H15F3O3), CH2Cl2 | C22H16F3O3 | C20H13F3O3 | C23H15F4O4 |
| MW/g mol–1 | 1057.79 | 385.35 | 358.30 | 431.35 |
| T/K | 298 | 298 | 150 | 298 |
| crystal system | orthorhombic | orthorhombic | monoclinic | monoclinic |
| space group | Pnma | Pna21 | P21/c | P21/c |
| a/Å | 20.3374(5) | 7.7794(3) | 9.3314 (10) | 13.227(4) |
| b/Å | 28.1705(7) | 19.3621(7) | 25.917 (3) | 6.4241(17) |
| c/Å | 8.7934(2) | 12.6018(4) | 13.5289 (15) | 22.762(6) |
| α (deg) | 90 | 90 | 90 | 90 |
| β (deg) | 90 | 90 | 106.616 (4) | 97.892(11) |
| γ (deg) | 90 | 90 | 90 | 90 |
| V/Å3 | 5037.9(2) | 18998.1(2) | 3135.2 (6) | 1915.9(9) |
| Z | 4 | 4 | 8 | 4 |
| Z′ | 1.5 | 1 | 2 | 1 |
| ρ/g cm–3 | 1.395 | 1.348 | 1.518 | 1.495 |
| μ/mm–1 | 1.938 | 0.926 | 1.075 | 0.127 |
| F(000) | 2184 | 796 | 1472.0 | 884 |
| radiation/Å | CuKα 1.54178 | CuKα 1.54178 | CuKα 1.54178 | MoKα 0.71073 |
| reflections collected | 35112 | 30831 | 88177 | 42888 |
| independent reflections | 5513 [R(int) = 0.1047] | 3865 [R(int) = 0.0580] | 6648 [R(int) = 0.0592] | 5570 [R(int) = 0.0413] |
| data/restraints/parameters | 5513/3/350 | 3865/1141/515 | 6648/0/481 | 5570/3/289 |
| goodness of fit on F2 | 1.036 | 1.051 | 1.044 | 1.045 |
| final R indices [I > σ(I)] | R1 = 0.0661, wR2 = 0.1653 | R1 = 0.0458, wR2 = 0.1115 | R1 = 0.0362, wR2 = 0.0918 | R1 = 0.0531, wR2 = 0.1136 |
| R índices (all data) | R1 = 0.1139, wR2 = 0.1978 | R1 = 0.0684, wR2 = 0.1384 | R1 = 0.0413, wR2 = 0.0958 | R1 = 0.0874, wR2 = 0.1288 |
Molecular Structures of I–VI by Single-Crystal X-ray Diffraction Studies
This section presents relevant intermolecular interactions of the compounds I–VI and some conformation similarities with other diols. Although there are no systematic studies of other three-ring-fused diols (for example, anthracene, acridine, phenazine, or xanthene), it is possible compare them with the simplest aromatic planar diol, resorcinol. Resorcinol can show polymorphism associated with its conformers. The polymorph α consists of molecules where the diols adopt an anti–anti conformation, while in polymorph β the hydroxyl groups adopt a syn–anti conformation.39 Additionally, a syn–syn conformer is frequently encountered in crystal engineering or cocrystals.40 Furthermore, the three possible conformations can be founded in the orcinol form II polymorph.41 Interestingly, despite their size, the TFXdiols structures can be considered as an extended series of orcinol because these conformations were found in their X-ray structures.
The xanthene molecules depart from planarity due to the sp3 oxygen atom that lies on the center of the fused rings. The concave shape can be described by θ, which is the angle created by the intersecting planes of the aromatic rings (Figure 3a). Table 2 contains the θ values found in each compound reported here. The angles θ range from 154.4° in I (the more concave structure) to 169.8° in IV (the more planar structure). In crystal I, each of the two molecules of the asymmetric unit has its own θ angle, with a difference of 12°.
Figure 3.

(a) Concave structure of TFXdiols exemplified by structure IV. The angle θ and the angle φ are indicated with double-headed arrows; see text for a detailed description. (b) Overlay of the crystallographic independent XTFdiols of the structures in this work. The related xanthenediol structure HORMUZ was included for a better comparison.31
Table 2. Compilation of θ and φ Angles in the Structures Discussed Herea.
| structure | θ [deg] | φ [deg] | |
|---|---|---|---|
| I | A | 166.85 | |
| B | 154.43 | ||
| II | A | 167.33 | 78.06 |
| B | 166.14 | 79.26 | |
| III | 161.31 | 69.58 | |
| IV | A | 169.81 | 78.34 |
| B | 168.45 | 69.18 | |
| V | 166.01 | 78.48 | |
| VI | 161.80 | 75.01 | |
Labels A and B refer to the crystallographically independent molecules.
Similarly, the torsion of the phenyl ring at C9 in structures II–VI can be described by φ, which indicates the angle between the relative position of this substituent and the closest aromatic ring in the xanthene framework. The values of φ range from 79.26° in III to 69.2° in IV. In the structure IV which contains two molecules in the asymmetric unit, φ differs by 10° in each molecule (Table 2).
To evaluate the similarity between all the molecular conformations and their molecular packings, we performed an in-depth analysis using CrystalCMP.42 This visualizing tool allowed us to confirm that the conformation is comparable across all compounds, despite the variations the θ and φ angles, as illustrated in the overlay of Figure 3b, with the root-mean-square values included in Figure S14.
Molecular Packing of TFXdiols I–III
After the conformational differences in the crystal structures were established, the intermolecular interactions of the TFXdiols will be described in this section. We start from structure I, and subsequently we will present the 3,6-xanthenediols II and III. The latter molecules differ from I because they feature a phenyl ring adjacent to the −CF3 moiety. It is important to note that structure II was previously reported by some of us,28 but it was included here because its molecular packing was not described in detail before.
The crystal of I was solved in
the orthorhombic system, Pnma space group. It contains
one and a half molecules of xanthenediol and a molecule of dichloromethane
(DCM) per asymmetric unit (Figure 4a). Despite being occluded within the crystalline lattice,
the DCM molecules do not establish strong intermolecular interactions
with the xanthenediol derivative. Conversely, the hydroxyl groups
in the TFXdiol adopt an anti–anti conformation and established
three 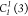 O–H···O
hydrogen
bonds with oxygen–oxygen distances d = 2.700(3),
2.792(3), and 2.773(3) Å and angles of 171(5)°, 169(5)°,
and 163(5)° respectively for the motif. Considering the length
and angles of these hydrogen bonds, they can be cataloged as moderate,43 with chains that extend over the three directions
of the unit cell (Figure 4b). The parameters of these bonds are compiled in Table 3 to provide a better
comparison for all the structures described here.
O–H···O
hydrogen
bonds with oxygen–oxygen distances d = 2.700(3),
2.792(3), and 2.773(3) Å and angles of 171(5)°, 169(5)°,
and 163(5)° respectively for the motif. Considering the length
and angles of these hydrogen bonds, they can be cataloged as moderate,43 with chains that extend over the three directions
of the unit cell (Figure 4b). The parameters of these bonds are compiled in Table 3 to provide a better
comparison for all the structures described here.
Figure 4.

(a) Hydrogen bonds in I that propagate the lattice in the three directions. (b) View down the c-axis of I.
Table 3. Bond Distances and Angles for the Interactions (O–H···O, O–H···π, C–H···π, and C–H···F) in I–VI.
| structure | # | H-bond | D–H (Å) | H···A (Å) | D···A (Å) | ∠DHA (deg) |
|---|---|---|---|---|---|---|
| I | 1 | O(12B)–H(12B)···O(12A) | 0.78(3) | 1.93(3) | 2.700(3) | 171(5) |
| 2 | O(12A)–H(12A)···O(11A) | 0.77(3) | 2.03(3) | 2.792(3) | 169(5) | |
| 3 | O(11A)–H(11A)···O(11B) | 0.79(3) | 2.01(3) | 2.773(3) | 163(5) | |
| II | 1 | O(12B)–H(12B)···O(20) | 0.88(3) | 1.80(3) | 2.670(3) | 171(3) |
| 2 | O(20)–H(20b)···O(11B) | 0.85(2) | 2.15(3) | 2.940(3) | 155(4) | |
| 3 | O(20)–H(20a)···O(12A) | 0.85(2) | 2.01(2) | 2.857(2) | 174(4) | |
| 4 | O(12A)–H(12A)···O(12B) | 0.86(3) | 1.97(3) | 2.776(2) | 155(2) | |
| 5 | O(11B)–H(11B)···π | 0.85(3) | 2.76(3) | 3.56(2) | 157(4) | |
| III | 1 | O(11)–H(11)···O(12) | 0.87(14) | 2.14(15) | 2.90(4) | 151(7) |
| 2 | O(12)–H(12)···O(11) | 1.07(11) | 1.70(11) | 2.77(2) | 172(8) | |
| IV | 1 | O(11B)–H(11B)···O(12A) | 0.84(2) | 1.98(2) | 2.742(2) | 151(2) |
| 2 | O(12A)–H(12A)···O(12B) | 0.89(2) | 1.96(2) | 2.814(2) | 161(2) | |
| 3 | O(12B)–H(12B)···O(11A) | 0.85(2) | 2.19(2) | 2.979(2) | 155(2) | |
| 4 | O(11A)–H(11A)···O(11B) | 0.84(2) | 2.00(2) | 2.819(2) | 164(2) | |
| 5 | C(6B)–H(6B)···F(A2) | 0.95(2) | 2.50(2) | 3.34(2) | 146(2) | |
| 6 | C(6A)–H(6A) ···Cg | 0.95(2) | 3.34(2) | 4.09(2) | 137(2) | |
| V | 1 | O(12)–H(12)···O(11) | 0.78(2) | 1.93(2) | 2.700(2) | 171(4) |
| 2 | O(11)–H(11)···π | 0.84(2) | 2.32(2) | 3.050(2) | 145(3) | |
| VI | 1 | O(23)–H(23)···O(12) | 0.886(18) | 1.88(2) | 2.763(2) | 175(3) |
| 2 | O(12)–H(12)···O(11) | 0.876(17) | 1.84(2) | 2.706(2) | 172(2) | |
| 3 | O(11)–H(11)···O(23) | 0.879(17) | 1.89(2) | 2.763(2) | 171(2) | |
| 4 | C(6)–H(6)···F(13b) | 0.93(2) | 2.63 | 3.473 | 150.6 |
The structure of crystal II was solved in a monoclinic system in the P21/c space group with two molecules per asymmetric unit. This disposition gave rise to both anti–syn and anti–anti conformations (Figure 5). Compared to the previous compound, the molecular packing of this concave molecule tends to form two types of head-to-tail pairs (labeled A and B). Pair A is created between molecules that are held together by means of weak π···π interactions, with a centroid–plane distance of 3.51 Å (Figure 5a).44 The pair B is held together by two hydrogen bonds (labeled 1 and 2) between −OH groups and adventitious water molecules. The donor–acceptor distances for the hydroxyl–water hydrogen bonds are 2.670(3) and 2.940(3) Å and O–H···O angles of 171(3)° and 155(4)° respectively (Figure 5b, Table 3). Pairs A and B are additionally interconnected through two hydrogen bonds (labeled 3 and 4) between them, with donor–acceptor distances 2.857(2) and 2.776(2) Å, and respective O–H···O angles of 174(4)° and 155(2)°. Finally, a O–H···π bond (labeled 5) with a donor–centroid acceptor distance of 3.56(2) Å and 157(4)° angle was also observed (Figure 5c and Table 3).45
Figure 5.

(a) Dimer A formed by π···π interactions in II. (b) Dimer B formed by bridging water molecules. (c) Hydrogen bonds between TFXdiol dimers.
Structure III belongs to an orthorhombic system, in
the space group Pna21, with one molecule
per asymmetric unit (Figure 2). The hydroxyl groups in this structure adopt an anti–syn
conformation producing infinite chains with two types of hydrogen
bonds  with donor–acceptor
internuclear
distances of 2.90(4) and 2.77(2) Å and angles of 151(7)°
and 172(8)° respectively (Figure 6a, Table 3). These chains propagate along the three crystallographic axes (Figure 6b).
with donor–acceptor
internuclear
distances of 2.90(4) and 2.77(2) Å and angles of 151(7)°
and 172(8)° respectively (Figure 6a, Table 3). These chains propagate along the three crystallographic axes (Figure 6b).
Figure 6.

(a) The two types of hydrogen bonds (1 and 2) that propagate the over the three directions in III. (b) Crystal packing of III viewed through the a-axis.
Interesting differences are observed between II and III. The hydrogen bonds in II only are propagated through the (010) plane, while in III they propagate in all three directions. This was attributed to the presence of water molecules in II that “block” the interactions in other directions and allow the presence of the two types of −OH conformers.
Molecular Packing of TFX-Diols IV–VI
After the structures I–III were analyzed, interactions of the hydroxyl
groups in
other positions of the xanthenediol framework can be better compared.
Structure IV with the hydroxyl groups in positions 2
and 7 was solved in a monoclinic system with a space group P21/c. It has two molecules
per asymmetric unit, and in both the hydroxyl groups adopt a syn–syn
conformation. In this structure, four types of hydrogen bonds give
rise to a 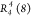 motif. These hydrogen
bonds show donor–acceptor
(oxygen–oxygen) distances of 2.742(2), 2.81(2), 2.979(2), 2.819(2)
Å and O–H···O angles of 151(2)°, 161(2)°,
155(2)°, and 164(2)° respectively (Table 3). Furthermore, CH···π
and CH···F weak interactions were identified, with
donor–centroid acceptor distances of 4.09 Å and donor–acceptor
distance of 3.34 Å, respectively (Figure 7a, Table 3).
motif. These hydrogen
bonds show donor–acceptor
(oxygen–oxygen) distances of 2.742(2), 2.81(2), 2.979(2), 2.819(2)
Å and O–H···O angles of 151(2)°, 161(2)°,
155(2)°, and 164(2)° respectively (Table 3). Furthermore, CH···π
and CH···F weak interactions were identified, with
donor–centroid acceptor distances of 4.09 Å and donor–acceptor
distance of 3.34 Å, respectively (Figure 7a, Table 3).
Figure 7.
(a) Ring motif,  , of IV, as well as
CH···π
and CH···F interactions. (b) Ring motif of V formed by hydroxyl hydrogen bonds. (c) Crystal packing of IV viewed through the b-axis. Layers in the
(101) plane are marked with green and orange colors. (d) Crystal packing
of V viewed through the b-axis. Layers
in the (100) plane are marked with green and orange colors.
, of IV, as well as
CH···π
and CH···F interactions. (b) Ring motif of V formed by hydroxyl hydrogen bonds. (c) Crystal packing of IV viewed through the b-axis. Layers in the
(101) plane are marked with green and orange colors. (d) Crystal packing
of V viewed through the b-axis. Layers
in the (100) plane are marked with green and orange colors.
We also include here the previously reported structure V to strengthen the comparison of this series.32 The hydroxyl groups in this molecule adopt a syn–syn conformation, suggesting that the methyl groups may interfere and prevent the motif found in IV. Instead, this structure shows an interesting pseudoring formed by two types of hydrogen bonds: two OH···O hydrogen bonds with a donor–acceptor distance of 2.700(2) Å and an angle of 171(4)°, and two OH···π bonds with a donor–centroid acceptor distance of 3.05(2) Å and an angle of 145(4)° (Figure 7b, Table 3). These interactions create layers along the (101) and (100) planes (Figure 7c,d).
Serendipitous Packing of VI
Finally, a
brief description of cocrystal VI will be provided. This
cocrystal was serendipitously obtained during the purification process
of compound 5, and it was included here to suggest that
the hydroxyl groups can be used in the future to engineering new cocrystal
platforms as have been widely implemented by another diols.46 This cocrystal was solved in a monoclinic system,
in the space group P21/c, with a xanthene molecule and half of hydroquinone molecule in the
asymmetric unit. Unlike structures IV and V, where the hydroxyl groups adopted syn–syn conformations,
the structure VI has an anti–syn conformation
that could be facilitated by the presence of its hydroquinone coformer.
This cocrystal shows a motif in which four xanthene molecules and
two hydroquinone molecules interact through three different O–H···O
bonds with donor–acceptor distances of 2.763(2), 2.706(2),
and 2.762(2) Å and angles of 175(3)°, 172(2)°, and
171(2)° respectively. These bonds give rise to a ring  , with layers in the plane
(100). The layers
propagate through the a-axis by a weak CH···F
interaction, with a donor–acceptor distance of 3.473 Å
and an angle of 150.6°. Furthermore, additional CF···π
weak interactions were observed with a distance between the fluorine
atom and the aromatic centroid of 3.241 Å, with an angle of 123.8°.47
, with layers in the plane
(100). The layers
propagate through the a-axis by a weak CH···F
interaction, with a donor–acceptor distance of 3.473 Å
and an angle of 150.6°. Furthermore, additional CF···π
weak interactions were observed with a distance between the fluorine
atom and the aromatic centroid of 3.241 Å, with an angle of 123.8°.47
Figure 8.

(a) Fourth-order ring motif of VI formed with hydroxyl hydrogen bonds. (b) Crystal packing of VI viewed through the b-axis.
Divergence of the Arrays of Compounds I–VI
After establishing the high degree of similarity among the conformation of the compounds I–VI, it was desirable to establish if the molecular packings could show some resemblances. To this end, CrystalCMP was employed again to provide a detailed comparison. The results clearly indicate that all packings are dissimilar (Figure S15), probably due to the presence of water or solvent molecules occluded within the lattice which could interfere with some intermolecular interactions.
Conclusions
We have described the synthesis of four new TFXdiols: 9-trifluoromethyl-3,6-xanthenediols I and II, as well as 9-trifluoromethyl-2,7-xanthenediols IV and VI. Their molecular structures were obtained through single-crystal X-ray diffraction studies and compared with the only two other examples found in the literature (molecules III and V). Despite the size and shape of the TFXdiols, their appended hydroxyl groups show conformations similar to those found in the smaller resorcinol. It was found that when the −OH groups are in positions 3 and 6, they adopt either anti–anti or anti–syn arrays to afford molecular packings where hydrogen bonds propagate across all directions. On the other hand, only syn–syn arrays are observed when the hydroxyl groups are in positions 2 and 7, giving rise to layers. Despite the differences in the relative positions of the OH groups, there is a high degree of similarity in the molecular conformations; however, the packings are considerably different. Finally, the analysis of structure VI indicates that the TFXdiols can be used as a platform to obtain new cocrystals in the future. We consider that the work presented here is timely because it provides the conformations and molecular packing of scarcely explored xanthenediols.
Acknowledgments
D.G.-B. thanks CONACYT for the scholarship (763328). This project was supported by CONACYT (A1-S-32820 and A1-S-17967, 251693) and PAPIIT (IN103920 and IA100321). The authors are in debt to O. J. Alvarez, E. R. Morales, and M. E. Hernandez for help with structural characterizations and to A. Lopez-Vivas and Alejandro Pompa for technical help.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06635.
Accession Codes
CCDC Deposition Nos. 2122963–2122966 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Present Address
§ PPG-Industries, Polymer Research Center, Tepexpan, Acolman, 55885, Estado de México, México
The authors declare no competing financial interest.
Supplementary Material
References
- Poupelin J. P.; Saint Ruf G.; Foussard Blanpin O.. Synthese et Proprietes Anti Inflammatoires de Derives Du Bis (Hydroxy 2 Naphtyl 1) Methane. I. Derives Monosubstitues. Eur. J. Med. Chem. 1978, 13 ( (1), ). [Google Scholar]
- Rewcastle G. W.; Atwell G. J.; Zhuang L.; Baguley B. C.; Denny W. A. Potential Antitumor Agents. 61. Structure-Activity Relationships for in Vivo Colon 38 Activity among Disubstituted 9-Oxo-9H-Xanthene-4-Acetic Acids. J. Med. Chem. 1991, 34 (1), 217–222. 10.1021/jm00105a034. [DOI] [PubMed] [Google Scholar]
- Wang H.; Lu L.; Zhu S.; Li Y.; Cai W. The Phototoxicity of Xanthene Derivatives against Escherichia Coli, Staphylococcus Aureus, and Saccharomyces Cerevisiae. Curr. Microbiol. 2006, 52 (1), 1. 10.1007/s00284-005-0040-z. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. K.; Rana K. C.; Mujahid M.; Sehar I.; Saxena A. K. Synthesis and in Vitro Study of 14-Aryl-14H-Dibenzo[a.j]Xanthenes as Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2009, 19 (19), 5590. 10.1016/j.bmcl.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Hafez H. N.; Hegab M. I.; Ahmed-Farag I. S.; El-Gazzar A. B. A. A Facile Regioselective Synthesis of Novel Spiro-Thioxanthene and Spiro-Xanthene-9′,2-[1,3,4]Thiadiazole Derivatives as Potential Analgesic and Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2008, 18 (16), 4538. 10.1016/j.bmcl.2008.07.042. [DOI] [PubMed] [Google Scholar]
- Saint-Ruf G.; Huynh-Trong-Hieu; Poupelin J. P. The Effect of Dibenzoxanthenes on the Paralyzing Action of Zoxazolamine. Naturwissenschaften 1975, 62 (12), 584. 10.1007/BF01166986. [DOI] [PubMed] [Google Scholar]
- Lin S.; Sin W. L. W.; Koh J. J.; Lim F.; Wang L.; Cao D.; Beuerman R. W.; Ren L.; Liu S. Semisynthesis and Biological Evaluation of Xanthone Amphiphilics as Selective, Highly Potent Antifungal Agents to Combat Fungal Resistance. J. Med. Chem. 2017, 60 (24), 10135–10150. 10.1021/acs.jmedchem.7b01348. [DOI] [PubMed] [Google Scholar]
- Sarma R. J.; Baruah J. B. One Step Synthesis of Dibenzoxanthenes. Dye. Pigment. 2005, 64 (1), 91. 10.1016/j.dyepig.2004.03.010. [DOI] [Google Scholar]
- Banerjee A.; Mukherjee A. K. Chemical Aspects of Santalin as a Histological Stain. Biotechnol. Histochem. 1981, 56 (2), 83. 10.3109/10520298109067286. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; King T. A.; Ko D. K.; Cha B. H.; Lee J. Performance and Photostability of Xanthene and Pyrromethene Laser Dyes in Sol-Gel Phases. J. Phys. D. Appl. Phys. 2002, 35 (13), 1473. 10.1088/0022-3727/35/13/303. [DOI] [Google Scholar]
- Bhowmik B. B.; Ganguly P. Photophysics of Xanthene Dyes in Surfactant Solution. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2005, 61 (9), 1997. 10.1016/j.saa.2004.07.031. [DOI] [PubMed] [Google Scholar]
- El-Brashy A. M.; El-Sayed Metwally M.; El-Sepai F. A. Spectrophotometric Determination of Some Fluoroquinolone Antibacterials by Binary Complex Formation with Xanthene Dyes. Farmaco 2004, 59 (10), 809. 10.1016/j.farmac.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Klimtchuk E.; Rodgers M. A. J.; Neckers D. C. Laser Flash Photolysis Studies of Novel Xanthene Dye Derivatives. J. Phys. Chem. 1992, 96 (24), 9817. 10.1021/j100203a044. [DOI] [Google Scholar]
- Deo C.; Abdelfattah A. S.; Bhargava H. K.; Berro A. J.; Falco N.; Farrants H.; Moeyaert B.; Chupanova M.; Lavis L. D.; Schreiter E. R. The HaloTag as a General Scaffold for Far-Red Tunable Chemigenetic Indicators. Nat. Chem. Biol. 2021, 17 (6), 718–723. 10.1038/s41589-021-00775-w. [DOI] [PubMed] [Google Scholar]
- Yao F. L.; Sheng S. R.; Jiang J. W.; Liu X. L.; Song C. S. Soluble Copoly(Aryl Ether Ether Ketone Ketone)s Containing Xanthene and Hexafluoroisopropylidene Moieties. J. Fluor. Chem. 2012, 144, 176. 10.1016/j.jfluchem.2012.09.007. [DOI] [Google Scholar]
- Fernandes J. A.; Morisaki Y.; Chujo Y. Aromatic Ring-Layered Polymer Containing 2,7-Linked Carbazole on Xanthene. Polym. Bull. 2010, 65 (5), 465. 10.1007/s00289-010-0353-3. [DOI] [Google Scholar]
- Morisaki Y.; Fernandes J. A.; Chujo Y. Xanthene-Based Oligothiophene-Layered Polymers. Macromol. Chem. Phys. 2010, 211 (22), 2407. 10.1002/macp.201000359. [DOI] [Google Scholar]
- Fernandes J. A.; Morisaki Y.; Chujo Y. Aromatic-Ring-Layered Polymers Composed of Fluorene and Xanthene. Polym. J. 2011, 43 (8), 733–737. 10.1038/pj.2011.58. [DOI] [Google Scholar]
- Chen Q.; Luo M.; Wang T.; Wang J. X.; Zhou D.; Han Y.; Zhang C. S.; Yan C. G.; Han B. H. Porous Organic Polymers Based on Propeller-like Hexaphenylbenzene Building Units. Macromolecules 2011, 44 (14), 5573. 10.1021/ma200915f. [DOI] [Google Scholar]
- Li X.; Zhang Y. C.; Zhao Y.; Zhao H. P.; Zhang B.; Cai T. Xanthene Dye-Functionalized Conjugated Porous Polymers as Robust and Reusable Photocatalysts for Controlled Radical Polymerization. Macromolecules 2020, 53 (5), 1550–1556. 10.1021/acs.macromol.0c00106. [DOI] [Google Scholar]
- Cai Z.; Liu Y.; Wang C.; Xie W.; Jiao Y.; Shan L.; Gao P.; Wang H.; Luo S. Ladder Polymers of Intrinsic Microporosity from Superacid-Catalyzed Friedel-Crafts Polymerization for Membrane Gas Separation. J. Membr. Sci. 2022, 644, 120115. 10.1016/j.memsci.2021.120115. [DOI] [Google Scholar]
- Zuo P.; Li Y.; Wang A.; Tan R.; Liu Y.; Liang X.; Sheng F.; Tang G.; Ge L.; Wu L.; Song Q.; McKeown N. B.; Yang Z.; Xu T. Sulfonated Microporous Polymer Membranes with Fast and Selective Ion Transport for Electrochemical Energy Conversion and Storage. Angew. Chem. 2020, 132 (24), 9651–9660. 10.1002/ange.202000012. [DOI] [PubMed] [Google Scholar]
- Tao L.; Yang H.; Liu J.; Fan L.; Yang S. Synthesis and Characterization of Highly Optical Transparent and Low Dielectric Constant Fluorinated Polyimides. Polymer (Guildf). 2009, 50 (25), 6009. 10.1016/j.polymer.2009.10.022. [DOI] [Google Scholar]
- Tao L.; Yang H.; Liu J.; Fan L.; Yang S. Synthesis of Fluorinated Polybenzoxazoles with Low Dielectric Constants. J. Polym. Sci. Part A Polym. Chem. 2010, 48 (21), 4668. 10.1002/pola.24253. [DOI] [Google Scholar]
- Volksen W.; Miller R. D.; Dubois G. Low Dielectric Constant Materials. Chem. Rev. 2010, 110 (1), 56. 10.1021/cr9002819. [DOI] [PubMed] [Google Scholar]
- Dhara M. G.; Banerjee S. Fluorinated High-Performance Polymers: Poly(Arylene Ether)s and Aromatic Polyimides Containing Trifluoromethyl Groups. Prog. Polym. Sci. 2010, 35, 1022–1077. 10.1016/j.progpolymsci.2010.04.003. [DOI] [Google Scholar]
- Olvera L. I.; Zolotukhin M. G.; Hernández-Cruz O.; Fomine S.; Cárdenas J.; Gaviño-Ramírez R. L.; Ruiz-Trevino F. A. Linear, Single-Strand Heteroaromatic Polymers from Superacid-Catalyzed Step-Growth Polymerization of Ketones with Bisphenols. ACS Macro Lett. 2015, 4 (5), 492. 10.1021/acsmacrolett.5b00164. [DOI] [PubMed] [Google Scholar]
- Olvera L. I.; Rodríguez-Molina M.; Ruiz-Trevino F. A.; Zolotukhin M. G.; Fomine S.; Cárdenas J.; Gavino R.; Alexandrova L.; Toscano R. A.; Martínez-Mercado E. A Highly Soluble, Fully Aromatic Fluorinated 3D Nanostructured Ladder Polymer. Macromolecules 2017, 50 (21), 8480–8486. 10.1021/acs.macromol.7b01413. [DOI] [Google Scholar]
- Groom C. R.; Bruno I. J.; Lightfoot M. P.; Ward S. C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno I. J.; Cole J. C.; Edgington P. R.; Kessler M.; Macrae C. F.; McCabe P.; Pearson J.; Taylor R. New Software for Searching the Cambridge Structural Database and Visualizing Crystal Structures. Acta Crystallogr. 2002, B58, 389–387. 10.1107/S0108768102003324. [DOI] [PubMed] [Google Scholar]
- Chen Y. H.; He J. B.; Bai X.; Li X. N.; Lu L. F.; Liu Y. C.; Zhang K. Q.; Li S. H.; Niu X. M. Unexpected Biosynthesis of Fluorescein-Like Arthrocolins against Resistant Strains in an Engineered Escherichia Coli. Org. Lett. 2019, 21 (16), 6499–6503. 10.1021/acs.orglett.9b02371. [DOI] [PubMed] [Google Scholar]
- Storch G.; Kim B.; Mercado B. Q.; Miller S. J. A Stereodynamic Redox-Interconversion Network of Vicinal Tertiary and Quaternary Carbon Stereocenters in Hydroquinone–Quinone Hybrid Dihydrobenzofurans. Angew. Chemie - Int. Ed. 2018, 57 (46), 15107–15111. 10.1002/anie.201808305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon G.; Coleman A. W.; Nassimbeni L. R.; Taljaard B. Inclusion of Aromatic Guests by a Xanthenol Host: Structures, Guest Exchange, and Desorption Kinetics. Cryst. Growth Des. 2005, 5 (6), 2331. 10.1021/cg049615o. [DOI] [Google Scholar]
- Curtis E.; Nassimbeni L. R.; Su H.; Taljaard J. H. Xanthenol Clathrates: Structures and Solid-Solid Reactions. Cryst. Growth Des. 2006, 6 (12), 2716. 10.1021/cg060384q. [DOI] [Google Scholar]
- da Silva M. L.; Teixeira R. R.; de Oliveira F. M.; de Moura Guimarães L.; Martins F. T. Vibrational Spectroscopic Studies, Theoretical Aspects, and X-Ray Analysis of Xanthenodiones (1,8-Dioxooctahydroxanthenes). J. Heterocycl. Chem. 2021, 58 (3), 777. 10.1002/jhet.4214. [DOI] [Google Scholar]
- Kavala V.; Murru S.; Patel B. K.; Das G. Self-Assembled Superstructure of Xanthene Derivatives. J. Chem. Crystallogr. 2007, 37 (8), 527. 10.1007/s10870-007-9201-1. [DOI] [Google Scholar]
- Bučar D. K.; Filip S.; Arhangelskis M.; Lloyd G. O.; Jones W. Advantages of Mechanochemical Cocrystallisation in the Solid-State Chemistry of Pigments: Colour-Tuned Fluorescein Cocrystals. CrystEngComm 2013, 15 (32), 6289. 10.1039/c3ce41013g. [DOI] [Google Scholar]
- Tao L.; Yang H.; Liu J.; Fan L.; Yang S. Synthesis and Characterization of Fluorinated Bisphenols and Tetraphenols via a Simple One-Pot Reaction. Synth. Commun. 2013, 43 (17), 2319–2325. 10.1080/00397911.2012.705214. [DOI] [Google Scholar]
- Safari F.; Olejniczak A.; Katrusiak A. Pressure-Dependent Crystallization Preference of Resorcinol Polymorphs. Cryst. Growth Des. 2019, 19 (10), 5629–5635. 10.1021/acs.cgd.9b00610. [DOI] [Google Scholar]
- MacGillivray L. R.; Reid J. L.; Ripmeester J. A. Supramolecular Control of Reactivity in the Solid State Using Linear Molecular Templates [4]. J. Am. Chem. Soc. 2000, 122 (32), 7817–7818. 10.1021/ja001239i. [DOI] [Google Scholar]
- Mukherjee A.; Grobelny P.; Thakur T. S.; Desiraju G. R. Polymorphs, Pseudopolymorphs, and Co-Crystals of Orcinol: Exploring the Structural Landscape with High Throughput Crystallography. Cryst. Growth Des. 2011, 11 (6), 2637–2653. 10.1021/cg200361x. [DOI] [Google Scholar]
- Rohlíček J.; Skořepová E.; Babor M.; Čejka J. CrystalCMP: An Easy-to-Use Tool for Fast Comparison of Molecular Packing. J. Appl. Crystallogr. 2016, 49 (6), 2172–2183. 10.1107/S1600576716016058. [DOI] [Google Scholar]
- Steiner T. The Hydrogen Bond in the Solid State. Angew. Chemie Int. Ed. 2002, 41 (1), 48–76. . [DOI] [PubMed] [Google Scholar]
- Yao Z.-F.; Wang J.-Y.; Pei J. Control of π–π Stacking via Crystal Engineering in Organic Conjugated Small Molecule Crystals. Cryst. Growth & Des. 2018, 18 (1), 7–15. 10.1021/acs.cgd.7b01385. [DOI] [Google Scholar]
- Schollmeyer D.; Shishkin O. V.; Ruhl T.; Vysotsky M. O. OH – p and Halogen – p Interactions as Driving Forces in the Crystal Organisations of Tri-Bromo and Tri-Iodo Trityl Alcohols. CrystEngComm 2008, 10, 715–723. 10.1039/b716442d. [DOI] [Google Scholar]
- Ramamurthy V.; Sivaguru J. Supramolecular Photochemistry as a Potential Synthetic Tool: Photocycloaddition. Chem. Rev. 2016, 116 (17), 9914–9993. 10.1021/acs.chemrev.6b00040. [DOI] [PubMed] [Google Scholar]
- Mooibroek T. J.; Gamez P.; Reedijk J. Lone Pair-π Interactions: A New Supramolecular Bond?. CrystEngComm 2008, 10 (11), 1501–1515. 10.1039/b812026a. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





