Abstract
The scallop shell waste (Pectinidae, one of saltwater clams) was used as a raw material (precursor) to prepare calcium lactate (Ca(C2H4OHCOO)2), and the physicochemical properties of scallop-derived calcium lactate were then investigated. The scallop waste was first ground to obtain calcium carbonate (CaCO3) powder, and the calcium lactate compounds were successfully synthesized by the reactions between shell-derived CaCO3 and lactic acid (C2H4OHCOOH). The short preparation time, high percentage yield, and low-cost production are the preferred manners, and, in this research, it was the reaction of 70 wt % lactic acid and scallop-derived CaCO3. The thermal decompositions of both CaCO3 precursor and all prepared calcium lactates resulted in the formation of calcium oxide (CaO), which is widely used as a catalyst for biodiesel production. By comparing with the literature, the results obtained from the characterization instruments (infrared spectrophotometer, X-ray diffractometer, thermogravimetric analyzer, and scanning electron microscope) confirmed the formation and crystal structure of both CaCO3 and its calcium lactate product. The morphologies of calcium lactate show different sizes depending on the acid concentration used in the reaction process. Consequently, this work reports an easy, uncomplicated, low-cost technique to change the cheap calcium compound product (scallop CaCO3) derived from shellfish waste to the valuable compound (calcium lactate), which can be used in many industries.
1. Introduction
Economic, social, and environmental sustainability is the most important for future globalization. Moreover, sustainable development is the development that is in line with the needs of both the present and the future.1 Nowadays, the development and commercialization of utilizing products obtained from biowaste materials are significantly increasing. Abundant and especially low-cost waste matter and its chemical conversion to useful compounds are key points in the broad field of green chemistry. In addition, the recycling of various biowastes is in line with many developing and developed countries to solve the environmental issues of the wastes, especially in Thailand where the bio-circular-green (BCG) economical model was established to change wastes to valuable products.2 Transformation of biowaste materials into value-added products shows a significant regional impact through the reduction of biowaste and biowaste treatment processes3 and can also add valuable revenue-generating streams through mass material production.
Aquaculture is an important occupation in many countries. It is widely designed to manage and control the organisms from both freshwater and marine sources.4 The three main aqua-cultured animals, i.e., fish, shrimp, and shellfish farms, are an essential protein source of both humans and other organisms. Shellfish production, especially bivalve shellfish, represents the main segment of the global seafood industry. Shellfish industries can be found in all coastal regions of many countries. Scallop is one of the well-known shellfishes, which is widely distributed throughout the world.5 Scallop aquaculture is one of the great economic importance, which supports both maricultural efforts and commercial fisheries. According to the National Oceanic and Atmospheric Administration (NOAA) Fisheries, about 23 000 tons of commercial landings of Atlantic Sea were observed in 2020 with the value of about 490 million USD. The information reported by the Food and Agriculture Organization of the United Nations (FAO) Fisheries and Aquaculture Department showed that more than 2 million tons of scallops have been consumed per year.6 However, the edible part of the scallop had only 10–16 wt %, resulting in about 84–90 wt % of scallop waste.7 This scallop food industry makes millions of tons of shellfish’s byproducts, which can be considered an interesting biowaste resource.8 The disposal of scallop shell wastes is turning into an increasingly serious issue for the marine aquaculture industries and various consumer countries.
Large numbers of shell wastes are conventionally dumped into landfills and/or public waters, which cause many environmental problems including health and sanitation issues, damage of the natural landscape, unpleasant smell as a consequence of the decomposition of organics attached to the shells, and management of public water surface as well as pollution to coastal fisheries.9−11 Consequently, some green technologies are required to find the methods to reduce the above-mentioned problems.12 For example, Borić et al. presented a sustainable and feasible platform for the extraction and purification of chitin (the second most abundant natural polysaccharide, after cellulose) from crustacean waste. Chitin was isolated from crustacean waste using a hybrid demineralization/dielectric barrier discharge (DBD) plasma process. Plasma and organic acids were applied to remove protein and inorganic minerals from the waste, respectively.13,14 Moreover, Bradić et al. presented a sustainable process for the recovery of value-added biomaterials from the abundant shrimp shell waste using natural deep eutectic solvents. Due to the properties of the selected solvent, chitin (98% purity) was separated from minerals and proteins in a single step.15
Calcium lactate, a white crystal salt with formula C6H10CaO6 or Ca(C2H4OHCOO)2, is composed of two lactate anions (CH3CHOHCO2–) for each calcium cation (Ca2+). It is available in several hydrate forms, and calcium lactate pentahydrate (C6H10CaO6·5H2O) is the most common one. Calcium lactate has been extensively applied in many industries. For medicine industries, it has been applied in humans and other organisms such as an antidote for soluble fluoride ingestion,16 hypocalcemia (calcium deficiencies),17 oral calcium administration for prevention of tetany, anti-tartar agent in some mouthwashes and toothpaste, antacid,18 and calcium source for preventing and treating calcium deficiencies.19 For food industries, it has been applied as a food additive with E number of E327 classified by the United States Food and Drug Administration (U.S. FDA) as Generally Recognized as Safe (GRAS),20 for uses as a stabilizer and thickener, nutritional supplement, leavening agent, flavor enhancer or flavoring agent, and firming agent.21 For plant and animal industries, it has been applied as a calcium source for animals and plants and used to maintain and extend the shelf life of flowers, fruits, and vegetables.22 For environmental industries, it is a coagulant for removing suspended solids from water23 because it is a renewable, nontoxic, and biodegradable compound.24 For chemical industries, it was used as a raw material for the production of advanced compounds such as lactic acid (C2H4OHCOOH) to use in food and medical fields,25 and calcium oxide (CaO) to use as a catalyst.26 For construction industries, it is mixed into the concrete to increase the compressive strength and reduce the water permeability, resulting in the formation of efficient bio-concrete.27 Because of the large applications of calcium lactate described above, consequently, it had been prepared by many preparation methods using starting agents such as lactic acid with nonrenewable ores such as calcium carbonate (CaCO3) and calcium hydroxide (Ca(OH)2).
The transformations of the shellfish wastes were recommended by the BCG model2 to transform waste into valuable calcium compounds such as calcium acetate and calcium lactate. Simple and rapid processes were also suggested in waste recycling. Recently, the recycling of biowastes is the strong intention. For example, crab shell,28 eggshells,29 littleneck clam (Ruditapes philippinarum) shell,30 and butter clam (Saxidomus purpuratus) shell31 were used as starting agents to synthesize calcium lactate. The difference in starting agents and preparation methods are the effects of the different physicochemical properties of the calcium lactate products. A large number of restaurants and many marine product manufacturers along Thailand’s coast discharge scallop shell as a waste, resulting in the increase of processing cost to exterminate the scallop waste.32
CaCO3 is the main composition (98 wt %) of scallop with some small interferences, which are magnesium carbonate (MgCO3, 0.79 wt %) and strontium carbonate (SrCO3, 0.15 wt %).33 Therefore, scallop shell was considered a suitable raw material for the production of calcium lactate. The objective of this work is to synthesize calcium lactate through a low-cost, rapid, and simple process using scallop shell as the precursor. The natural form of scallop shell was first cleaned and milled to obtain the scallop-derived CaCO3 powder. After that, the reaction between scallop-derived CaCO3 powder with lactic acid was employed to prepare the calcium lactate. The difference in the synthesis conditions may influence the characteristics of the synthesized product. Therefore, the effect of some operational parameters, i.e., the concentration of lactic acid used in the synthesis process, on the percentage yield, drying time, reaction temperature, and other physicochemical properties of calcium lactate, were also investigated. All investigated parameters were then used to point out and obtain the suitable parametric value that synthesizes a low-cost calcium lactate product. Scallop-derived CaCO3 powder and all synthesized calcium lactate products were analyzed by several scientific methods including X-ray fluorescence (XRF), X-ray diffraction (XRD), infrared (IR) adsorption, thermogravimetry/derivative thermogravimetry (TG/DTG), and scanning electron microscopy (SEM) to prove that the obtained target compounds were CaCO3 and calcium lactate.
2. Results and Discussion
2.1. Production Results
Table 1 illustrates the preparation conditions, percentage yields, and production cost of calcium lactate monohydrate (Ca(C2H4OHCOO)2·H2O) produced from the reaction between scallop-derived CaCO3 and various lactic acid concentrations. They show an increase in the temperature of the exothermic reaction as the concentration of lactic acid increases. These reaction temperatures are the highest temperature that occurred during the reaction. As described in Section 4.2, the preparation of calcium lactate using higher concentrations of lactic acid leads to the completely dried powder within a short time. For example, completely dried powders were obtained at the exposure time of about 3 h for CaLT-40, but for CaLT-70, it took only 30 min. The percentage yields of the calcium lactate product obtained from four reactions were found in the range of 89–93%, which were not significantly different, with the highest production yield observed when 50 wt % lactic acid was employed to prepare calcium lactate. The cost of the production process with consideration of only raw materials for every four reactions was found to be closely same (0.1366–0.1405 USD·kg–1). In terms of production results, short preparation time, high percentage yield, and low production cost are the preferred characteristics, and, in this research, it was the reaction between 70 wt % lactic acid and scallop-derived CaCO3 labeled as CaLT-70.
Table 1. Preparation Conditions, Percentage Yields, and Production Cost of Calcium Lactate (CaLT) Monohydrate (Ca(C2H4OHCOO)2·H2O) Produced from the Reaction between Scallop-Derived CaCO3 Powders and Various Lactic Acid Concentrations.
| compound | lactic acid concentration (wt %) | percentage yield (%) | dried time | reaction temperature (°C) | production cost (USD·kg–1) |
|---|---|---|---|---|---|
| CaLT-40 | 40 | 92.68 | 3 h | 36 | 0.1405 |
| CaLT-50 | 50 | 93.49 | 2 h | 47 | 0.1390 |
| CaLT-60 | 60 | 89.86 | 45 min | 57 | 0.1390 |
| CaLT-70 | 70 | 90.49 | 0.30 min | 64 | 0.1366 |
2.2. X-ray Fluorescence
A Bruker SRS 3400 XRF spectrometer was employed to characterize all synthesized compounds. Table 2 presents the chemical compositions of scallop-derived CaCO3 and its calcium lactate products. Calcium oxide (CaO) and oxygen (O) are the major chemical composition of CaCO3. However, some trace impurities such as magnesium (Mg), sulfur (S), phosphorus (P), and potassium (K) were also observed. Four synthesized calcium lactate samples (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) mainly contain calcium oxide (69.56–70.88%) and oxygen (26.50–29.30%) contents and minor elemental impurities (S, K, P, Mg, and Na). The S and P elements of about <1% may come from calcium sulfate and calcium phosphate, which were impurities in scallop shells. The presence of SO42– and PO43– ions may be confirmed by vibrational bands in the Fourier transform infrared (FTIR) spectrophotometry results. For the elemental quantities of calcium and oxygen contents in calcium lactate products, the theoretical values are 71.43% CaO and 28.57% O, which are close to the observed values of all synthesized calcium lactate products. Based on elemental content, the purities of calcium lactates were estimated and found to be in the range of 97.38–99.23%. The highest amount of the elemental impurities was observed in CaLT-60 followed by CaLT-40. Nevertheless, the accumulation of toxic elements in scallop shells may occur depending on the source. Therefore, the elemental compositions should be investigated before each use. According to the results, calcium lactates derived from scallop shell waste obtained in this work did not have the contents of toxic elements such as chromium (Cr), strontium (Sr), cadmium (Cd), arsenic (As), lead (Pb), chlorine (Cl), fluorine (F), etc. From the obtained XRF data, the lowest amount of impurities was observed from the CaLT-70 sample, pointing out that the lactic acid with a concentration of 70 wt % should be used as the reagent to prepare calcium lactate with the highest purity.
Table 2. Chemical Compositions and Purities of Raw-CaCO3 Starting Material and Scallop-Derived Calcium Lactate (CaLT) Prepared at Different Lactic Acid Concentrations.
| compound | chemical
composition (%) |
purity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | O | Na | Mg | S | P | K | summation | ||
| CaCO3 | 69.92 | 27.5 | 0.14 | 0.59 | 0.25 | 1.6 | 100 | 97.88 | |
| CaLT-40 | 70.20 | 29.2 | 0.61 | 100 | 98.27 | ||||
| CaLT-50 | 70.04 | 29.3 | 0.66 | 100 | 98.06 | ||||
| CaLT-60 | 69.56 | 28.2 | 0.83 | 0.24 | 0.82 | 0.36 | 100 | 97.38 | |
| CaLT-70 | 70.88 | 26.5 | 0.54 | 0.45 | 1.63 | 100 | 99.23 | ||
2.3. Thermal Analysis
A PerkinElmer Pyris Diamond TGA instrument was applied to confirm the identity of calcium carbonate (CaCO3) obtained from the scallop shell waste and its calcium lactate monohydrate (Ca(C2H4OHCOO)2·H2O) products. The TG and DTG curves as presented in Figure 1 show the thermal decomposition behavior of scallop-derived CaCO3 sample by presenting the decomposition temperature range of the sample. The TG mass loss and DTG peak were found to have one thermal decomposition period (single step) of about 600–800 °C. Moreover, according to the TG curve, the remaining calcium lactate mass was 57.97%. A DTG peak at 843 °C with the DTG value of −0.159 μg·min–1 was assigned to the elimination of a carbon dioxide molecule.
Figure 1.

Thermal decomposition behavior of raw-CaCO3 starting material derived from scallop shell waste.
It is well known that the products of the thermal decomposition of CaCO3 are solid-state CaO and gas-state CO2.3 The compositions of thermodecomposed CaO and CO2 products of approximately 57.97 and 43.03 wt % were observed, respectively.3 The result indicates clearly that the hypochlorite treatment in the process of the scallop shell preparation can remove the organic material. Consequently, eq 1 can be used to present the thermal decomposition of scallop-derived CaCO3 powder.
| 1 |
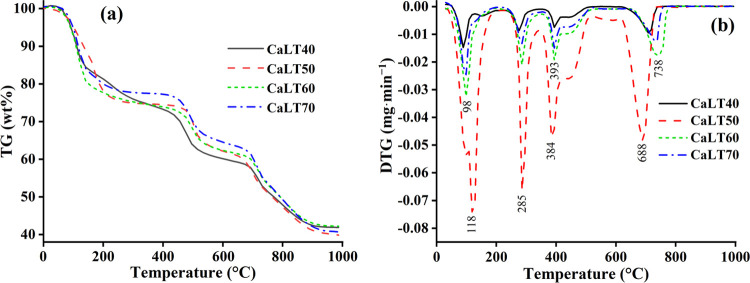
The thermal decompositions of calcium lactate monohydrate products were also investigated. Figure 2a,b, respectively, shows the TG and DTG thermograms of all calcium lactate samples (CaLT-40, CaLT-50, CaLT-60, and CaLT-70). The mass loss percentages in the range of 30–1000 °C as shown in the TG curves related well to the DTG peaks, indicating the decomposition mechanism of thermal reactions. Obviously, the patterns of the TG and DTG curves of CaLT-40, CaLT-50, CaLT-60, and CaLT-70 were similar. Three steps of the mass losses observed in TG curves for all calcium lactates appeared in the range of 100–200, 200–600, and 600–800 °C. Their corresponding DTG peaks at about 98–118, 285, 384–393, and 688 °C related to the elimination of water H2O (dehydration process), ethyl 2-hydroxypropanoate (C5H10O3 or CH3CHOHCOOC2H5), and carbon dioxide CO2 (decarbonation process), respectively. The total mass loss was about 58%, which corresponded to the retained mass of about 42%. Therefore, the thermal decomposition mechanism of scallop-derived Ca(C2H4OHCOO)2·H2O resulted in the formation of calcium oxide (CaO), which can be described in the following equations (eqs 2–4).
Figure 2.
Thermal decomposition behaviors of CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations: (a) TG curves and (b) DTG curves.
First step: Elimination of H2O (dehydration process)
| 2 |
Second step: Elimination of ethyl lactate (CH3CHOHCOOC2H5)
| 3 |
Final step: Elimination of CO2 (decarbonation process)
| 4 |
The thermal decomposition of each calcium lactate monohydrate was additionally described in detail. The TG and DTG curves of the CaLT-40 sample show the temperature range and the mass loss with three thermal decomposition steps. The first step is in the range of 100–300 °C. The corresponding DTG peak at 89 °C with a DTG peak value of about −0.0148 μg·min–1 is assigned as the loss of adsorbed water that existed on the surface of the synthesized Ca(C2H4OHCOO)2·H2O sample, whereas the DTG peak at 275 °C with DTG peak value of about −0.0087 μg·min–1 is the dehydration process, resulting in the formation of calcium lactate anhydrous (Ca(C2H4OHCOO)2). The second step is in the range of 350–500 °C. Its DTG peak at 393 °C with a DTG peak value of about −0.0075 μg·min–1 is the decomposition of Ca(C2H4OHCOO)2 to the CaCO3. The final step is in the range of 600–700 °C. Its DTG peak at 708 °C with a DTG peak value of about −0.0095 μg·min–1 is the decarbonation process, and CaO was thermally formed. The total mass loss to obtain the final thermal decomposition product (CaO) was 58.11%.
The TG and DTG curves of CaLT-50 show similar results to the CaLT-40. Three thermal decomposition steps were observed. The DTG peaks of the first step (20–250 °C) at 118 °C with a DTG peak value of −0.0742 μg·min–1 are assigned to the loss of adsorbed water that existed on the surface of Ca(C2H4OHCOO)2·H2O, whereas the DTG peak at 285 °C (DTG peak at −0.0663 μg·min–1) is the dehydration process to form Ca(C2H4OHCOO)2. The DTG peak of the second step (250–550 °C) at 384 °C (DTG peak at −0.0471 μg·min–1) is the decomposition of Ca(C2H4OHCOO)2 to form CaCO3. The DTG peak of the final step (600–700 °C) at 688 °C (DTG peak at −0.0482 μg·min–1) is the decarbonation process to form CaO. The total mass loss was 60.16%. Three thermal decomposition steps of CaLT-60 as shown in the TG and DTG curves were also observed. The DTG peaks of the first step (100–300 °C) at 99 °C (DTG peak at −0.0324 μg·min–1) are assigned to the loss of adsorbed water, whereas the DTG peak at 286 °C (DTG peak at −0.0210 μg·min–1) is the dehydration process. The DTG peak of the second step (350–500 °C) at 394 °C (DTG peak at −0.0192 μg·min–1) is the decomposition of Ca(C2H4OHCOO)2. The DTG peak of the final step (600–700 °C) at 738 °C (DTG peak at −0.0176 μg·min–1) is the decarbonation process. The total mass loss was 57.78%. According to the TG and DTG curves, CaLT-70 also exhibited three thermal decomposition steps. The DTG peaks at 99 °C (DTG peak at −0.0246 μg·min–1, first step, 100–300 °C) is assigned to the loss of adsorbed water, whereas the DTG peak at 286 °C (DTG peak at −0.0134 μg·min–1) is the dehydration process. The DTG peak at 394 °C (DTG peak at −0.0150 μg·min–1, second step, 350–500 °C) is the decomposition of Ca(C2H4OHCOO)2. The last DTG peak at 728 °C (DTG peak at −0.0122 μg·min–1, final step, 600–700 °C) is the decarbonation process. The total mass loss was 59.28%. Therefore, based on the results of the TGA technique as shown in Figure 2, the results confirmed the identity of calcium lactate monohydrate (Ca(C2H4OHCOO)2·H2O), which was successfully synthesized by the reaction between scallop-derived CaCO3 powder and lactic acid at the concentrations of 40, 50, 60, and 70 wt %.
2.4. Infrared Spectroscopy
A PerkinElmer Spectrum GX FTIR spectrophotometer was used to investigate the vibrational characteristics of scallop-derived CaCO3 (precursor) and its calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70). Figure 3 presents the infrared adsorption results of the precursor and calcium lactates prepared by various lactic acid concentrations (40, 50, 60, and 70 wt %). The adsorption spectrum of the CaCO3 precursor as shown in Figure 3a indicates the vibrational characteristic of carbonate (CO32–) anion that is presented in the crystal structure of CaCO3.34−36 Some dominating adsorption peaks were explained in detail. An absorption peak at about 1440 cm–1 was assigned to the asymmetric stretching mode of the C–O bond, which is the major adsorption characteristic of CO32–. An infrared peak at about 1801 cm–1 is described as the vibrational stretching mode of the carbonyl C=O group. A vibrational position at about 875 cm–1 is the symmetric stretching mode of the C–O bond, whereas a vibrational position at about 712 cm–1 is the out-of-plane and in-plane bending of CO32–. Two vibrational bands at about 2523 and 2924 cm–1 were the combination and overtone of the symmetric and asymmetric stretching modes of the C–O bond, which are the vibrational characteristics of the CO32– of aragonite polymorph of CaCO3.3,37 The bands in the wavenumber range of 3010–3680 cm–1 are assigned to the vibrational characteristics of both symmetric and asymmetric stretching modes of C–H of methyl (−CH3) group and O–H of water (H2O) that were physically adsorbed on the sample surface.
Figure 3.
Infrared adsorption spectrum of (a) scallop =0derived CaCO3 and (b) CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations.
Figure 3b presents the infrared adsorption spectra of CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations. Obviously, the obtained infrared spectra are similar for all calcium lactates. The broad bands of the infrared adsorption region of about 3030–3740 cm–1 were assigned to the O–H stretching mode of H2O (in both symmetric and asymmetric modes) in the crystal structure of Ca(C2H4OHCOO)2·H2O. The adsorption peaks at about 1746, 1573, and 1487 cm–1 were attributed to the stretching mode of the carbonyl C=O group. As described by Pavia et al.,38 the vibrational mode of the C=O bond was observed at the adsorption wavenumber range of 1850–1650 cm–1. In addition, the results observed in this research are in line with the results reported by Lee and Kim39 and Cheong.40 Lee and Kim prepared calcium lactate using black snail (Semisulcospira libertina (bensoni)) as the CaCO3 precursor, and the obtained black-snail-derived calcium lactate was then characterized by the infrared adsorption technique.39 They reported that the peaks at 1592 and 1430 cm–1 were assigned to the asymmetric and symmetric stretching modes of the carboxylate RCOO– functional group. The weak adsorption bands at about 3030–2852 cm–1 and about 1515–1340 cm–1 were assigned to the stretching and bending modes of the C–H bond. The adsorption bands at about 1131–1000 and 670–600 cm–1 could be assigned to ν3 and ν4 modes of the SO42– and/or PO43–anions, which are observed in the same regions.36 This result is consistent with the presence of S and P elemental impurities in the XRF results
2.5. X-ray Diffraction
An X-ray diffractometer with the Cu Kα X-ray light source was used to investigate and confirm the crystal structure of the samples prepared in this research. The diffraction pattern of the synthesized compound that was prepared from the scallop shell is shown in Figure 4a, pointing out the diffraction characteristics of calcium carbonate (CaCO3). The obtained diffraction patterns of CaCO3 powder exhibit the calcite polymorph according to the PDF #47-1743 database.41 The diffraction positions (2θ) at 23.01, 29.34, 31.35, 35.93, 39.44, 43.16, 47.07, 47.42, 48.44, 56.49, 56.62, 57.32, and 57.47° are the characteristic of calcite CaCO3 structure.3,42 In addition, the 2θ value at 29.34 corresponded to the hkl plane of 104, which is the main characteristic of calcite CaCO3 polymorph with a trigonal crystal system.43 The space group of calcite CaCO3 are R3®c. The lattice parameters are a = 4.97630 Å and c = 17.0904 Å, whereas the lattice volume is 366.520 Å3.44 The bond lengths between Ca–O and C–O are 2.3574 and 1.2836 Å, respectively.45Figure 4b shows the diffraction pattern of CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations. The pattern results of all obtained products were compared with those of calcium lactate reported in the literature,19,46,47 which exhibited the diffraction characteristics of calcium lactate (Ca(C2H4OHCOO)2·H2O). The XRD patterns of CaLT-50, CaLT-60, and CaLT-70 samples are quite similar, which are slightly different from those of CaLT-40, observed at around 11°. This indicates that the amorphous phase of calcium acetate monohydrate of a CaLT-40 is different from solid phases of other samples.46,47 All obtained patterns of CaLT samples are in good agreement with the reference data19,46,48 and agree with observations from IR and TGA.
Figure 4.
X-ray diffraction (XRD) patterns of (a) raw-CaCO3 starting material derived from scallop shell waste and (b) CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations.
2.6. Morphology
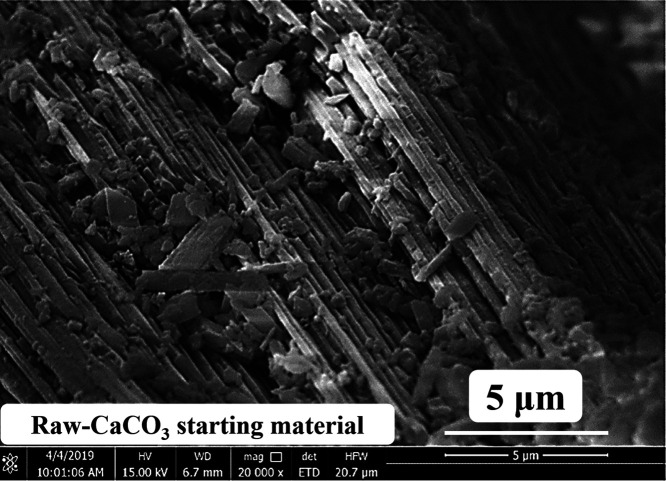
LEO VP1450 SEM was used to investigate the morphological characteristics of the prepared CaCO3 starting material derived from scallop shell waste and the CaCO3-derived calcium lactate products (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) prepared at different lactic acid concentrations. The results of the morphological identity at a magnification of 20,000 times showed that the SEM image of the scallop-derived CaCO3 as shown in Figure 5 had a prism-like structure, together with islands (about 5 μm in size), which were probably a result of the broken shell.
Figure 5.
Scanning electron microscopy (SEM) image of raw-CaCO3 starting material derived from scallop shell waste.
From the previous research, calcium lactate was used in the pharmaceutical application as a calcium source for preventing and treating calcium deficiencies because of its good solubility and bioavailability.19 Therefore, the morphology and crystal size of the synthesized calcium lactate product were also characterized because the morphology and crystal size of the product may influence its performance after applying in the pharmaceutical field. The morphological identity at 2000 times magnification shown in Figure 6 revealed that calcium lactate monohydrate in four samples obtained from CaCO3 reacted with lactic acid differed in both shape and size according to the concentrations of the reacting acid (40, 50, 60, and 70 wt %). The calcium lactate monohydrates synthesized from the shell-derived CaCO3 material and 40 (Figure 6a) or 50 (Figure 6b) wt % lactic acid were found to be similar. They were thin, overlapping, and shapeless sheets (about 50 μm in size). On the other hand, when 60 wt % lactic acid was used as the reagent to prepare calcium lactate monohydrate, the irregularly agglomerated hexagonal rods (approximately 50 μm in size) shown in Figure 6c were observed. In the case of 70 wt % lactic acid, the rectangular shape shown in Figure 6d is arranged in an orderly manner. These rectangular crystals did not clump together and were approximately 30 μm in size.
Figure 6.
Scanning electron microscopy (SEM) images of CaCO3-derived calcium lactate products ((a) CaLT-40, (b) CaLT-50, (c) CaLT-60, and (d) CaLT-70) prepared at different lactic acid concentrations.
3. Conclusions
Calcium lactate, which can be applied in various applications, was successfully synthesized from the reaction between scallop-derived calcite CaCO3 precursor and various lactic acid concentrations (40, 50, 60, and 70 wt %). This work presented some valuable information for the calcium lactate production using biowaste as starting material. This shell-derived CaCO3 preparation can be beneficially used to replace the usages of natural lime or dolomite ores obtained from non-living things which are limited resources. Through an experimental design, the efficiency of the preparation of calcium lactate was studied by considering the effect of lactic acid concentration on the reaction temperature, time consumption, and production yield observed from the calcium lactate production. It was found that the reaction temperature increased with increasing lactic acid concentration, and 70 wt % of lactic acid is the suitable concentration to use in the calcium lactate preparation. The single and triple steps were observed from the thermal decomposition of scallop-derived CaCO3 and its calcium lactate products, respectively, and CaO was the final thermal decomposition product. All applying characterization techniques confirmed that the reaction product obtained from scallop shell powders was calcium lactate monohydrate (Ca(C2H4OHCOO)2·H2O). The infrared adsorption and X-ray diffraction results of the synthesized calcium lactates are well consistent with those of previous reports. The morphologies of the calcium lactates observed from the electron microscope exhibited differences in both shapes and sizes, which depended on the lactic acid concentrations.
4. Experimental Section
4.1. Raw Material Preparations
Scallop shell waste (20 kg) was collected from a shell dumping place in Bangpoo district (location: 13.517 457 591 626 444, 100.654 937 526 486 28), Samut Prakarn province, Thailand; 14% sodium hypochlorite aqueous solution (density: 1.21 g·mL–1) was used to clean the scallop waste, and the cleaning process was finished when the meat and dust particles of the scallop were removed completely. The cleaned waste was dried at 100 °C for 1 h using an oven. After that, the cleaned and dried waste was ground to obtain scallop-derived CaCO3 powders and then sieved with 100 meshes, obtaining approximately 140 μm of powder particle size. Using this preparing process, the cost of scallop-derived CaCO3 powders is no more than 0.03 USD per kg. The prepared CaCO3 powder was then used as a low-cost starting material for calcium lactate synthesis.
Industrial-grade lactic acid (88 wt % C2H4OHCOOH, concentrated solution) was also employed without further purification as another starting reagent to synthesize the calcium lactate. This concentrated acid was first diluted with distilled water to prepare four different concentrations of lactic acids (40, 50, 60, and 70 wt %). It is well known that the strongly exothermic process was observed during the dilution procedure. Therefore, before further use, the diluted acid solutions were left to cool down. The prices of 40, 50, 60, and 70 wt % lactic acid solutions were calculated and found to be approximately 2.07, 2.58, 3.10, and 3.62 USD·kg–1, respectively.
4.2. Calcium Lactate Production
The target compounds, calcium lactate Ca(C2H4OHCOO)2·nH2O with different physicochemical compositions and properties, were synthesized based on the following chemical reaction as shown in eq 5
| 5 |
The synthesis method of calcium lactate was described as follows. Four beakers for each lactic acid concentration (40, 50, 60, and 70 wt %) were used to mix the starting reactants for the synthesis of calcium lactate products. Each beaker contained 10 g of scallop-derived CaCO3 powder. The first beaker was immediately added with 37 mL of 40 wt % lactic acid. The mixture was then stirred continuously for 5 min using a stirring grass rod. The mixing reaction was an exothermic process, whereas carbon dioxide (CO2) (reacted product) and water (reaction medium) were released and evaporated simultaneously. Using this preparation technique, calcium lactate was formed. The obtained mixture was then exposed to ambient conditions (air environment and 1 atm) without applying any other temperature for about 3 h to dry itself, and the completely dried powder was obtained. The synthesized calcium lactate product using 40 wt % lactic acid was designated and labeled as CaLT-40. For the other three beakers, the processes were repeated by replacing 40 wt % lactic acid by 50, 60, and 70 wt % lactic acids using the acid volumes of 30, 25, and 22 mL, respectively. The completely dried powders were obtained at the exposure time of about 2 h, 45, and 30 min, and the products were labeled as CaLT-50, CaLT-60, and CaLT-70, respectively. The production yield of calcium lactate according to eq 5 was calculated using eq 6
| 6 |
where mobs and mtheor are the mass of the obtained calcium lactate powder in the preparation process from each lactic acid concentration and the mass of the theoretical calcium lactate product, respectively.
4.3. Characterization
Chemical contents and impurities of starting material (scallop waste-derived CaCO3) and four synthesized calcium lactate (CaLT-40, CaLT-50, CaLT-60, and CaLT-70) samples were characterized and identified by an SRS 3400 X-ray fluorescence spectrometer (XRF, Bruker). The crystal structure and the purity of the synthesized compounds were identified by an X-ray powder diffractometer (XRD; Bruker AXS) with the Cu Kα spectral line (λ = 1.54056 Å) as the incident radiation. The resulting XRD patterns were compared to the Powder Diffraction File (PDF) database49 created by the International Centre for Diffraction Data (ICDD) for the identification of the compounds synthesized in this research. The 2θ angles, ranged from 10 ot 60° for CaCO3 and from 5 to 60° for calcium lactate, were measured with a scan speed of 1 s per step at a 0.01° increment to increase the reliability of the experimental data.50 Infrared adsorption spectra were recorded by a Spectrum GX Fourier transform infrared spectrophotometer (FTIR, PerkinElmer) from 4000 to 400 cm–1 with 16 scans at a resolution of 4 cm–1. To prepare the sample for infrared adsorption rest, 1 mg of each sample was homogeneously mixed with 10 mg of potassium bromide (KBr, spectroscopic grade) powder.50 A Pyris Diamond thermogravimetric analyzer (TGA, PerkinElmer) was implemented to investigate the thermal decomposition behavior of the sample. Thermogravimetric (TG) and differential thermogravimetric (DTG) curves of samples were achieved from these thermodecomposed experiments. The experiment was operated under nitrogen (N2) gas (99.9% purity) from room temperature to 900 °C at a heating rate of 10 °C·min–1. Approximately 7 mg of the sample was filled into an alumina pan without both sample pressing process and a pan lid, and the thermodecomposed curves were recorded using calcined α alumina oxide (α-Al2O3) as the reference material.51 Finally, surface morphologies of all synthesized compounds were observed by a LEO VP1450 scanning electron microscope (SEM, Zeiss) using the gold-coated sputtering technique.
Acknowledgments
This work was supported by the Thailand Science Research and Innovation (TSRI) (RE-KRIS/008/64). The authors thank the Scientific Instruments Center KMITL for supporting TGA, FTIR, XRD, and SEM techniques.
The authors declare no competing financial interest.
References
- Dugmore T. I. J.; Clark J. H.; Bustamante J.; Houghton J. A.; Matharu A. S. Valorisation of biowastes for the production of green materials using chemical methods. Top. Curr. Chem. 2017, 375, 46 10.1007/s41061-017-0133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiksin J. EU and Thailand address bio-circular-green-economy. MRS Bull. 2020, 45, 419. 10.1557/mrs.2020.175. [DOI] [Google Scholar]
- Sronsri C.; Sittipol W.; Kongpop U. Performance of CaO catalyst prepared from magnetic-derived CaCO3 for biodiesel production. Fuel 2021, 304, 121419 10.1016/j.fuel.2021.121419. [DOI] [Google Scholar]
- Jegatheesan V.; Shu L.; Visvanathan C.. Aquaculture Effluent: Impacts and Remedies for Protecting the Environment and Human Health. In Encyclopedia of Environmental Health, Nriagu J. O., Ed.; Elsevier: Burlington, 2011; pp 123–135. [Google Scholar]
- Chantler P.; Parsons G.; Shumway S., Scallops: Biology, Ecology and Aquaculture. 2nd ed.; Elsevier Science, 2006. [Google Scholar]
- Bianchi M.; Chopin F.; Farme T.; Franz N.; Fuentevilla C.; Garibaldi L.; Laurenti A., FAO: The State of World Fisheries and Aquaculture. 2014.
- Berik N.; Çankırılıgil E. C. Determination of proximate composition and sensory attributes of scallop (Flexopecten glaber) gonads. Mar. Sci. Tech. Bull. 2013, 2, 5–8. [Google Scholar]
- Sawai J. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 2011, 16, 95–102. 10.4265/bio.16.95. [DOI] [PubMed] [Google Scholar]
- Yan N.; Chen X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. 10.1038/524155a. [DOI] [PubMed] [Google Scholar]
- Miculescu F.; Mocanu A.-C.; Maidaniuc A.; Dascălu C.-A.; Miculescu M.; Voicu I.; Ciocoiu R.-C.. Biomimetic Calcium Phosphates Derived from Marine and Land Bioresources. In Hydroxyapatite - Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets, Thirumalai J., Ed.; IntechOpen, 2018. [Google Scholar]
- Vecchio K. S.; Zhang X.; Massie J. B.; Wang M.; Kim C. W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. 10.1016/j.actbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Pavlas M.; Dvořáček J.; Pitschke T.; Peche R. Biowaste treatment and waste-to-energy—Environmental benefits. Energies 2020, 13, 1994. 10.3390/en13081994. [DOI] [Google Scholar]
- Borić M.; Puliyalil H.; Novak U.; Likozar B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. 10.1039/C7GC03735J. [DOI] [Google Scholar]
- Borić M.; Vicente F. A.; Jurković D. L.; Novak U.; Likozar B. Chitin isolation from crustacean waste using a hybrid demineralization/DBD plasma process. Carbohydr. Polym. 2020, 246, 116648 10.1016/j.carbpol.2020.116648. [DOI] [PubMed] [Google Scholar]
- Bradić B.; Novak U.; Likozar B. Crustacean shell bio-refining to chitin by natural deep eutectic solvents. Green Process. Synth. 2019, 9, 13–25. 10.1515/gps-2020-0002. [DOI] [Google Scholar]
- Ullah R.; Zafar M. S.; Shahani N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman K.; McDaniel L.; Molloy M.. The Harriet Lane Handbook E-Book; Elsevier Health Sciences, 2020. [Google Scholar]
- Feldman E. C.; Nelson R. W.; Reusch C.; Scott-Moncrieff J. C.. Canine and Feline Endocrinology - E-Book; Elsevier health sciences, 2014. [Google Scholar]
- Mititelu M.; Morosan E.; Nicoară A. C.; Secăreanu A. A.; Musuc A. M.; Atkinson I.; Pandele Cusu J.; Nitulescu G. M.; Ozon E. A.; Sarbu I.; Balaci T. D. Development of immediate release tablets containing calcium lactate synthesized from black sea mussel shells. Mar. Drugs 2022, 20, 45. 10.3390/md20010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Additives E. P. o.; Feed P. oS. uiA.; Rychen G.; Aquilina G.; Azimonti G.; Bampidis V.; Bastos M. dL.; Bories G.; Chesson A.; Cocconcelli P. S.; Flachowsky G. Safety of lactic acid and calcium lactate when used as technological additives for all animal species. EFSA J. 2017, 15, e04938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daengprok W.; Garnjanagoonchorn W.; Mine Y. Fermented pork sausage fortified with commercial or hen eggshell calcium lactate. Meat Sci. 2002, 62, 199–204. 10.1016/S0309-1740(01)00247-9. [DOI] [PubMed] [Google Scholar]
- Linares-Morales J. R.; Gutiérrez-Méndez N.; Rivera-Chavira B. E.; Pérez-Vega S. B.; Nevárez-Moorillón G. V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustainable Food Syst. 2018, 2, 50. 10.3389/fsufs.2018.00050. [DOI] [Google Scholar]
- Devesa-Rey R.; Fernández N.; Cruz J.; Moldes A. Optimization of the dose of calcium lactate as a new coagulant for the coagulation–flocculation of suspended particles in water. Desalination 2011, 280, 63–71. 10.1016/j.desal.2011.06.051. [DOI] [Google Scholar]
- Syafaat F. Y.; Yusuf Y. Influence of Ca/P concentration on hydroxyapatite (HAp) from Asian moon scallop shell (Amusium pleuronectes. Int. J. Nanoelectron. Mater. 2019, 12, 357–362. [Google Scholar]
- Cao X.; Lee H.-J.; Yun H. S.; Koo Y.-M. Solubilities of calcium and zinc lactate in water and water-ethanol mixture. Korean J. Chem. Eng. 2001, 18, 133–135. 10.1007/BF02707210. [DOI] [Google Scholar]
- Abdullah R.; Abdullah A. Z. In Effect of Catalyst to Glycerol Ratio in the Production of Lactic Acid via Hydrothermal Reaction Using Calcium Oxide and Strontium Oxide Catalysts, AIP Conf. Proc.; AIP Publishing LLC, 2018; p 020197.
- Vijay K.; Murmu M. Effect of calcium lactate on compressive strength and self-healing of cracks in microbial concrete. Front. Struct. Civ. Eng. 2019, 13, 515–525. 10.1007/s11709-018-0494-2. [DOI] [Google Scholar]
- Han X.; Wang C.; Yang X.; Wang B.; Sang Y.; Sun J. Study on preparation of calcium lactate and chitin from crab shell. Food Res. Dev. 2018, 39, 65–70. [Google Scholar]
- CHEN X.; ZHANG Y.; ZHAO Y.-e. Study on preparation of calcium lactate from eggshell by hydrothermal synthesis. China Food Add. 2010, 1, 191–194. [Google Scholar]
- Park S. H.; Jang S. J.; Lee H. J.; Lee G.-W.; Lee J. K.; Kim Y. J.; Kim J.-S.; Heu M. S. Optimization of calcium acetate preparation from littleneck clam (Ruditapes philippinarum) shell powder and its properties. Korean J. Food Sci. Technol. 2015, 47, 321–327. 10.9721/KJFST.2015.47.3.321. [DOI] [Google Scholar]
- Yoon I. S.; Lee G.-W.; Lee H. J.; Park S. H.; Park S. Y.; Lee S. G.; Kim J.-S.; Heu M. S. Characterization of calcium lactate prepared from butter clam Saxidomus purpuratus shell powder. Korean J. Fish Aquat. Sci. 2016, 49, 301–309. 10.5657/kfas.2016.0301. [DOI] [Google Scholar]
- Guan G.; Chen G.; Kasai Y.; Lim E. W. C.; Hao X.; Kaewpanha M.; Abuliti A.; Fushimi C.; Tsutsumi A. Catalytic steam reforming of biomass tar over iron-or nickel-based catalyst supported on calcined scallop shell. Appl. Catal., B 2012, 115–116, 159–168. 10.1016/j.apcatb.2011.12.009. [DOI] [Google Scholar]
- Buasri A.; Worawanitchaphong P.; Trongyong S.; Loryuenyong V. Utilization of scallop waste shell for biodiesel production from palm oil–optimization using Taguchi method. APCBEE Proc. 2014, 8, 216–221. 10.1016/j.apcbee.2014.03.030. [DOI] [Google Scholar]
- Chung K.-H.; Jung S.-C.; Park B.-G. Eco-friendly deicer prepared from waste oyster shells and its deicing properties with metal corrosion. Environ. Technol. 2020, 42, 3360–3368. 10.1080/09593330.2020.1729243. [DOI] [PubMed] [Google Scholar]
- Seesanong S.; Laosinwattana C.; Boonchom B. Microparticles of calcium carbonate CaCO3, calcium hydrogen phosphate hydrate CaHPO4·1.9H2O and tricalcium phosphate Ca3(PO4)2 prepared from golden apple snail shells (Pomacea canaliculata). Res. J. Chem. Environ. 2020, 24, 1–6. [Google Scholar]
- Seesanong S.; Laosinwattana C.; Chaiseeda K.; Boonchom B. A simple and rapid transformation of golden apple snail (Pomacea canaliculata) shells to calcium carbonate, monocalcium and tricalcium phosphates. Asian J. Chem. 2019, 31, 2522–2526. 10.14233/ajchem.2019.22173. [DOI] [Google Scholar]
- Buasri A.; Chaiyut N.; Loryuenyong V.; Worawanitchaphong P.; Trongyong S. Calcium oxide derived from waste shells of mussel, cockle, and scallop as the heterogeneous catalyst for biodiesel production. Sci. World J. 2013, 2013, 460923 10.1155/2013/460923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia D. L.; Lampman G. M.; Kriz G. S.; Vyvyan J. A.. Introduction to Spectroscopy; Cengage Learning, 2014. [Google Scholar]
- Lee Y.-K.; Kim S.-D. Preparation and characteristics of calcium lactate from black snail. Prev. Nutr. Food Sci. 2003, 8, 166–172. 10.3746/jfn.2003.8.2.166. [DOI] [Google Scholar]
- Cheong S. H. Physicochemical properties of calcium lactate prepared by single-phase aragonite precipitated calcium carbonate. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1786–1794. [Google Scholar]
- Wang Y.; Lu D. Study on oral ulcer powder using temperature-dependent X-ray diffraction technique. Top. Chem. Mater. Eng. 2018, 1, 104–106. [Google Scholar]
- Munawaroh F.; Muharrami L. K.; T; Arifin Z. Synthesis and characterization of precipitated CaCO3 from ankerite prepared by bubbling method. KnE Eng. 2019, 2019, 98–104. 10.18502/keg.v1i2.4435. [DOI] [Google Scholar]
- Sronsri C.; Kongpop U.; Sittipol W. Quantitative analysis of calcium carbonate formation in magnetized water. Mater. Chem. Phys. 2020, 245, 122735 10.1016/j.matchemphys.2020.122735. [DOI] [Google Scholar]
- Sitepu H.; Al-Ghamdi R. A. Quantitative phase analysis of XRD data of sludge deposits from refineries and gas plants by use of the rietveld method. Adv. X-Ray Anal. 2019, 62, 45–57. [Google Scholar]
- Antao S. M.; Hassan I.; Wang J.; Lee P. L.; Toby B. H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. 10.3749/canmin.46.5.1501. [DOI] [Google Scholar]
- d’Aertrycke J.-B. dM.Development of a crystallization process for calcium lactate recovery from a fermentation broth. PhD Thesis, Catholic University of Louvain: Louvain-la-Neuve, Wallonia, Belgium, 2020. [Google Scholar]
- Sakata Y.; Shiraishi S.; Otsuka M. Characterization of dehydration and hydration behavior of calcium lactate pentahydrate and its anhydrate. Colloids Surf., B 2005, 46, 135–141. 10.1016/j.colsurfb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kim J.-M.; Chang S. M.; Kim I.-H.; Koo Y.-M.; Hong H.; Kim W.-S. Drowning-out crystallization of calcium lactate for crystal size control. Korean Chem. Eng. Res. 2009, 47, 740–746. 10.1021/ie8007488. [DOI] [Google Scholar]
- Fawcett T. G.; Kabekkodu S.; Blanton J.; Blanton T. Chemical analysis by diffraction: the Powder Diffraction File. Powder Diffr. 2017, 32, 63–71. 10.1017/S0885715617000288. [DOI] [Google Scholar]
- Sronsri C.; Boonchom B. Synthesis, characterization, vibrational spectroscopy, and factor group analysis of partially metal-doped phosphate materials. Spectrochim. Acta, Part A 2018, 194, 230–240. 10.1016/j.saa.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Sronsri C. Thermal dehydration kinetic mechanism of Mn1.8Co0.1Mg0.1P2O7·2H2O using Málek’s equations and thermodynamic functions determination. Trans. Nonferrous Met. Soc. China 2018, 28, 1016–1026. 10.1016/S1003-6326(18)64739-9. [DOI] [Google Scholar]