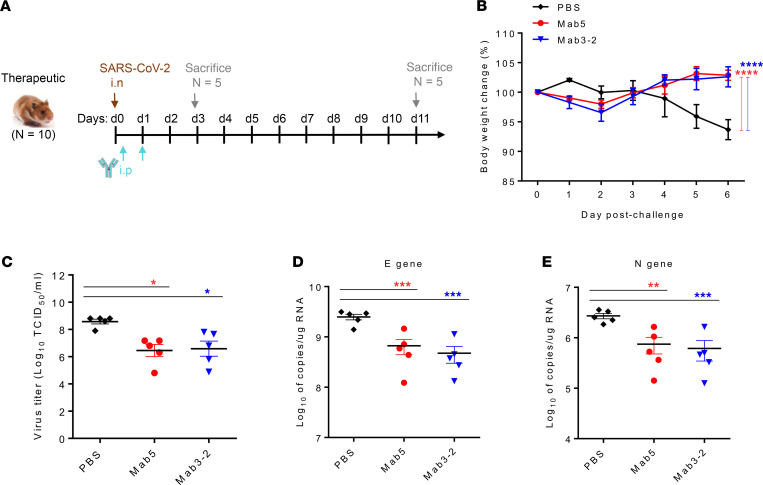
Figure 3. Protective efficacy of neutralizing mAbs against SARS-CoV-2 infection in Syrian hamsters.
(A). SARS-CoV-2 challenge model for determining the therapeutic efficacy. Each group (n = 10) was challenged intranasally with 105 TCID50 of SARS-CoV-2. Each hamster was intraperitoneally injected with 2.5 mg (16.5 mg/kg) of neutralizing mAb at 3 hours and again at 1 day after infection, and in parallel, control hamsters were injected with saline. The percent body weight change was recorded over 11 days. (B) For determination of the therapeutic efficacy of murine mAbs, the animals were treated with Abs by intraperitoneal injection at 3 hours and again at 24 hours after infection. The percent body weight change was recorded daily over 11 days. (C) The infectious viral load in the lung tissues (n = 5) on day 3 was quantified by TCID50 assay. (D and E) The viral loads were determined by RT-qPCR targeting 2 SARS-CoV-2 genes (labeled E and N). The data were statistically analyzed by (B) 2-way ANOVA followed by Tukey multiple-comparison test and (C–E) ordinary 1-way ANOVA followed by Tukey multiple-comparison test. All data are reported as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with the control.