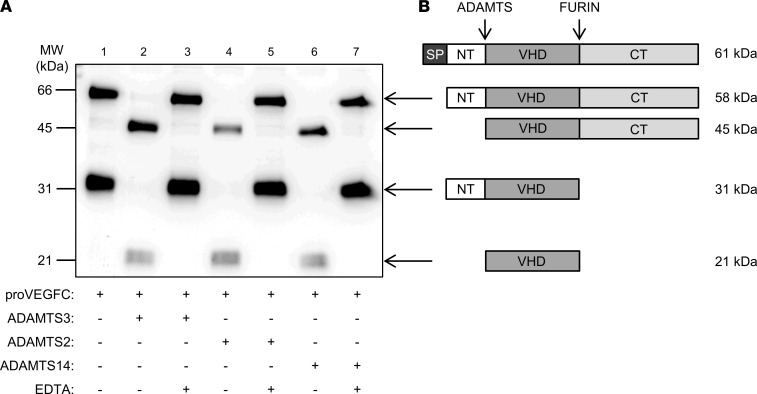
Figure 1. Processing of human pro-VEGFC by ADAMTS3, ADAMTS2, and ADAMTS14 proteases.
(A) Conditioned medium from HEK293 cells expressing full-length pro-VEGFC was incubated with buffer (as negative control, lane1), ADAMTS3 (as positive control, lane 2), ADAMTS2, or ADAMTS14, in the presence or absence of EDTA used as inhibitor. The electrophoretic pattern of VEGFC was analyzed by Western blotting in reducing conditions. In absence of active enzymes (lane 1, 3, 5, and 7), VEGFC can be detected as a 58 kDa form (full-length pro-VEGFC without signal peptide) and a 31 kDa form generated by C-terminal processing by furin. In the presence of active ADAMTS3 (lane 2), ADAMTS2 (lane 4), and ADAMTS14 (lane 6), the 58 kDa form was totally converted into a 45 kDa polypeptide, whereas the 31 kDa form was processed into the fully mature 21 kDa VEGFC, which is in line with N-terminal processing of VEGFC proteins. (B) Schematic illustration of the different VEGFC forms, with their molecular weights provided (SP, signal peptide; NT, N-terminal propeptide; VHD, VEGF homology domain; CT, C-terminal propeptide).