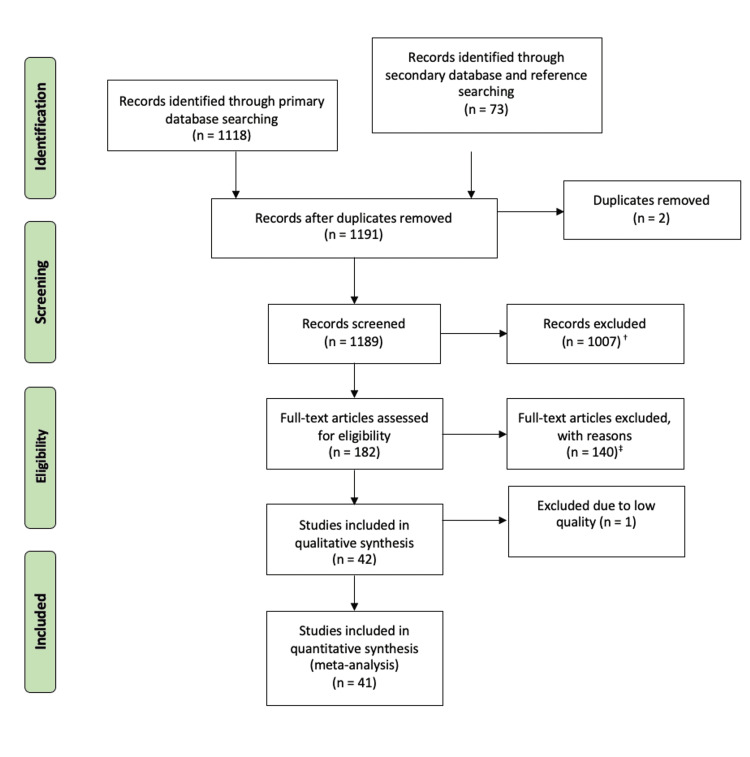
Figure 1. The Study Selection Process Following the PRISMA Flowchart.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis. Original image created by the authors.
†Study/trial unrelated to research question: 583; study/trial focussing on stroke risk factors: 36; study/trial focussing on management of stroke: 24; study/trial focussing on cardiac pathologies: 283; study/trial focussing on atrial fibrillation: 35; study/trial focussing on cardiorenal syndromes: 5; study/trial focussing on coronavirus disease 2019 (COVID-19): 30; study/trial focussing on sickle cell disease: 8; study/trial focussing on artificial intelligence in disease: 3
‡Review article/meta-analysis/editorial: 34; full text not available, only abstract: 6; study on stroke risk/chronic strokes/transient ischaemic attacks: 15; study on biomarkers and mainly other diseases in stroke: 20; study on differentiating stroke types and stroke mimics: 7; study focussing on diagnosis/management/clinical decision-making: 28; study determining risk of acute outcomes: 9; study to determine pathophysiology of disease: 7; study criteria not specific: 2; animal/laboratory-based studies (validation): 11; study looking at cost associated with stroke: 1