Figure 2. Prexasertib Pharmacokinetics in Pediatric Patients with Recurrent or Refractory Solid and CNS Tumors.
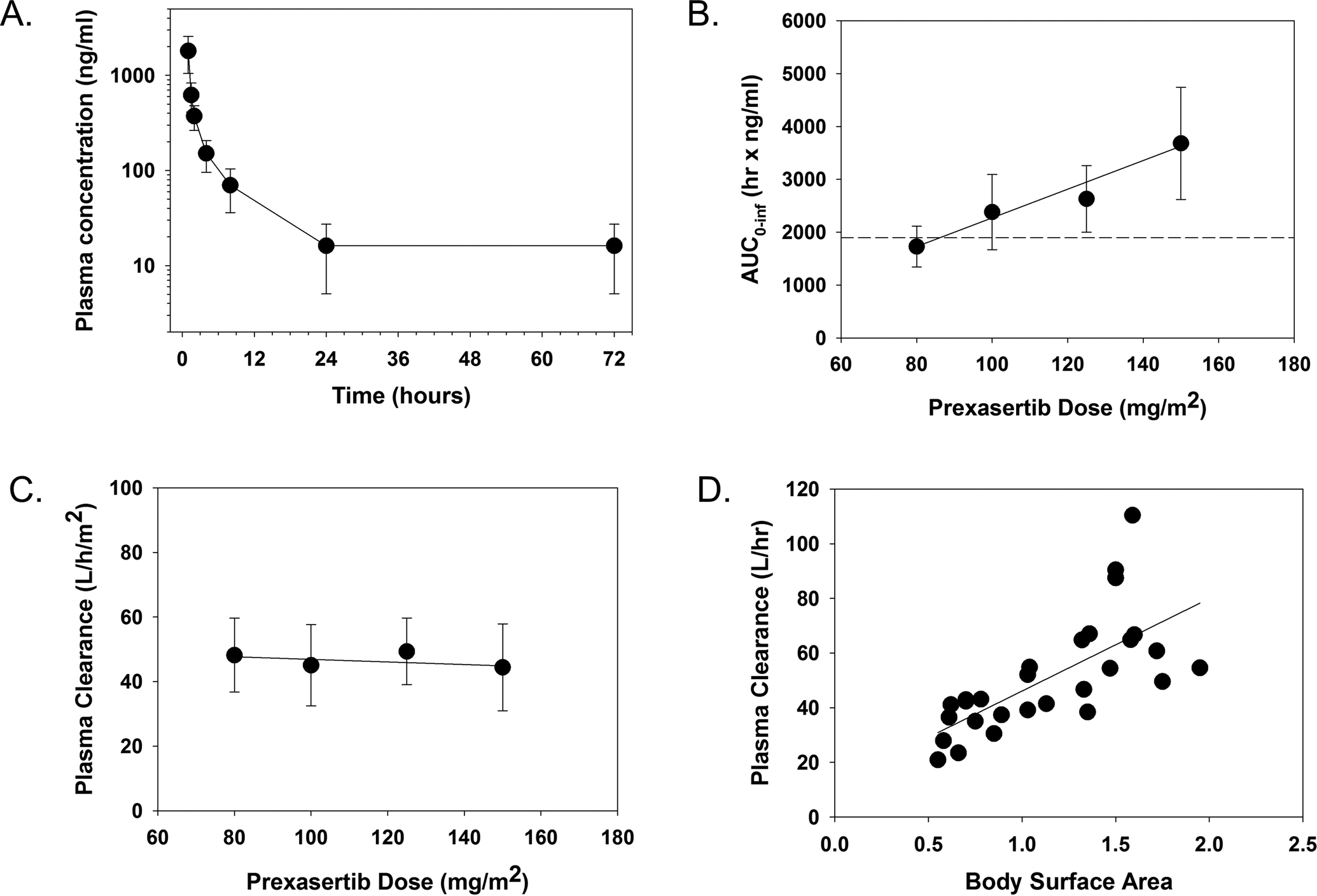
The mean plasma concentration as a function of time for patients treated with 150 mg/m2 of prexasertib (A), mean AUC0−∞ for each dose level with horizontal line on the plot representing median AUC0–72h value (1896 ng*hr/ml) predicted for efficacy based on the Calu-6 preclinical PK/PD model11 (B), mean plasma clearance for each dose level (C), and plasma clearance as a function of body surface area (D). Error bars represent the standard deviation for each observation.
