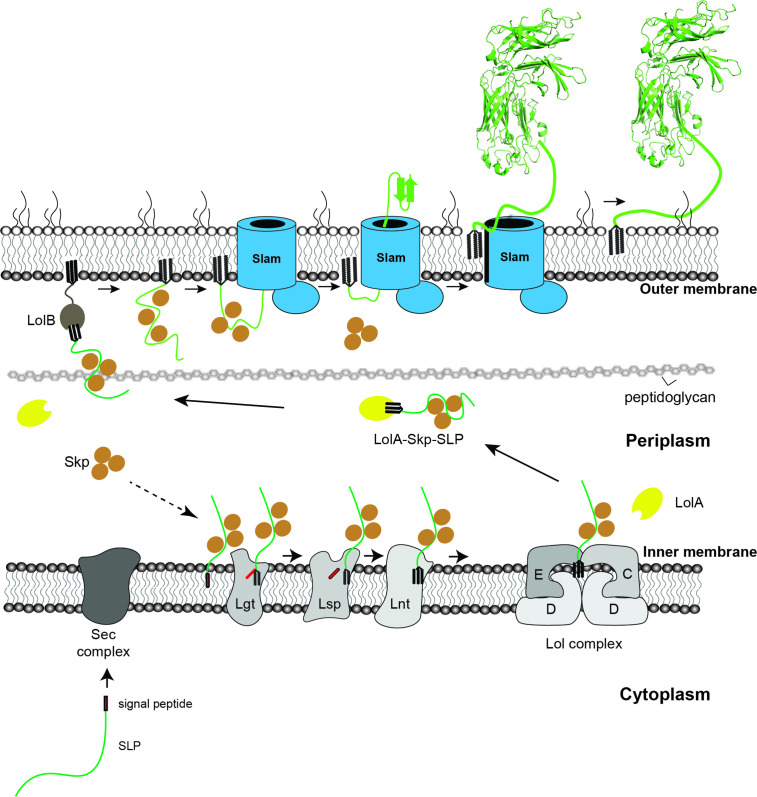
Figure 6. Proposed model of localization for Slam-dependent surface lipoproteins in Gram-negative bacteria.
Once the surface lipoproteins emerged from the Sec complex, periplasmic chaperone Skp binds to the SLPs to prevent early folding while their N-terminus is modified and lipidated by Lgt, Lsp, and Lnt before getting passed to the Lol complex. LolA then releases lipidated SLPs to the periplasm while Skp stays bound to prevent SLPs folding. LolB in the outer membrane (OM) might serve as the receiver for the LolA–SLP–Skp complex. The ‘specificity motif’ present at the C-terminus of the unfolded SLP is recognized by Slam and then transported across the outer membrane through the Slam channel and folds on the other side. Once the entire length of the SLP is transported, the Slam lateral gate allows the lipid anchor to ‘flip’ from the inner leaflet to the outer leaflet of the outer membrane.