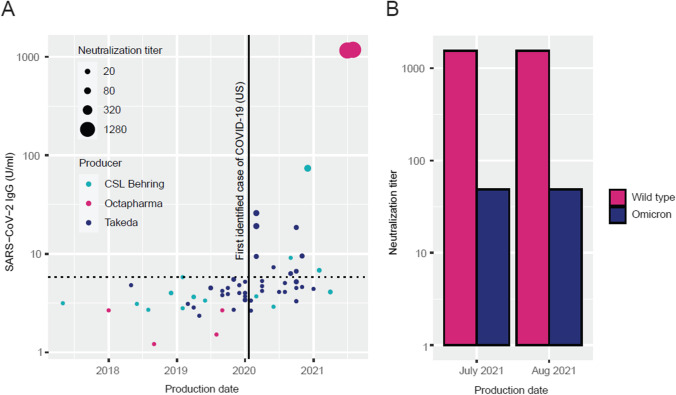
Fig. 1.
The emergence of neutralizing antibodies against wild-type and Omicron variants of SARS-CoV-2 in commercial immunoglobulin products. A Anti-SARS-CoV-2 spike protein antibody concentration and neutralizing capacity in consecutive batches of commercial immunoglobulin products used in clinical practice at the Immunodeficiency Unit at Karolinska University Hospital in relation to production date. The size of the dots represents the highest dilution (titer) at which the individual batch is effective in a microneutralization assay using wild-type/Wuhan strain live SARS-CoV-2. The producers are represented by different colors. In pre-pandemic batches (left of vertical line) no antibody concentration above 5.8 U/ml was observed, which is represented by a dashed horizontal line. Concentrations and titers have been adjusted to compensate for differences in total IgG concentration (100–200 g/l) in the included products. B The two immunoglobulin batches with the highest concentration of antibodies and neutralizing capacity against wild-type SARS-CoV-2 were also tested for cross-reactive neutralization capacity against the Omicron variant of SARS-CoV-2