Figure 1.
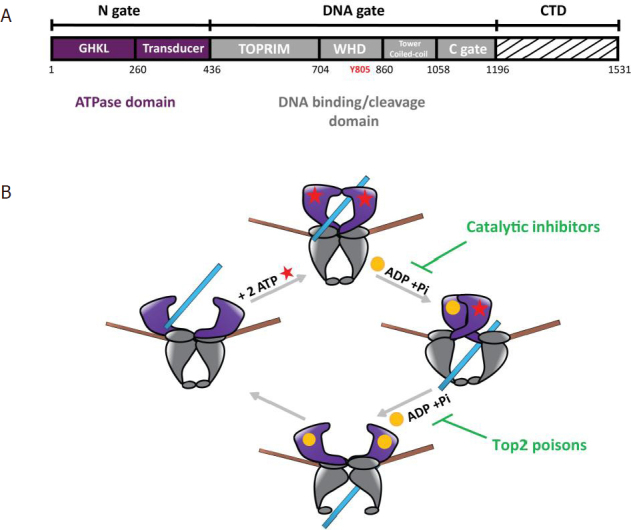
Domain organization of Type 2 DNA topoisomerase and catalytic cycle. A: domain organization of the human Top2 enzymes. The Top2 enzymes are composed of three dimeric interfaces. The N-gate (in purple) is composed of a GHKL domain binding ATP and a transducer domain. The DNA-gate (in grey) comprises the WHD with the conserved catalytic tyrosine (Y805 in red) and the Toprim domain, that contains three acidic residues binding magnesium ions. The C-gate forms a dimeric interface where the DNA exits the enzyme at the end of the reaction. The CTD (dashed lines) differs between species and between the human isoforms Top2α and Top2β. Residue numbering is that of the Top2α isoform. B: catalytic cycle of Top2 relaxation activity. (1) The Top2 enzyme binds a DNA G-segment (in brown) at the dimeric interface at the DNA-gate. (2) Upon ATP binding (red stars), a second DNA fragment called T-segment (in blue) is trapped in the ATPase domain. (3) Introduction of a reversible DNA double strand break in the G-segment is coupled to the hydrolysis of an ATP molecule, leading to the release of ADP (yellow sphere) and an inorganic phosphate, and results in the transport of the T-segment through the break. Catalytic inhibitors such as bispiperazine (ICRF derivatives) trap a closed clamp intermediate, affecting ATP-driven conformational changes of the allosteric assembly. (4) Hydrolysis of a second ATP molecule triggers the release of the T-segment through the C-gate and resets the enzyme. Top2 poisons such as etoposide and doxorubicin prevent G-segment religation. Top2: type 2 DNA topoisomerase; CTD: C-terminal domain; WHD: winged helix domain; G: gated; T: transported; GHKL: Gyrase, Hsp90, Histidine Kinase, MutL domain; TOPRIM: topoisomerase-primase domain
