Figure 4.
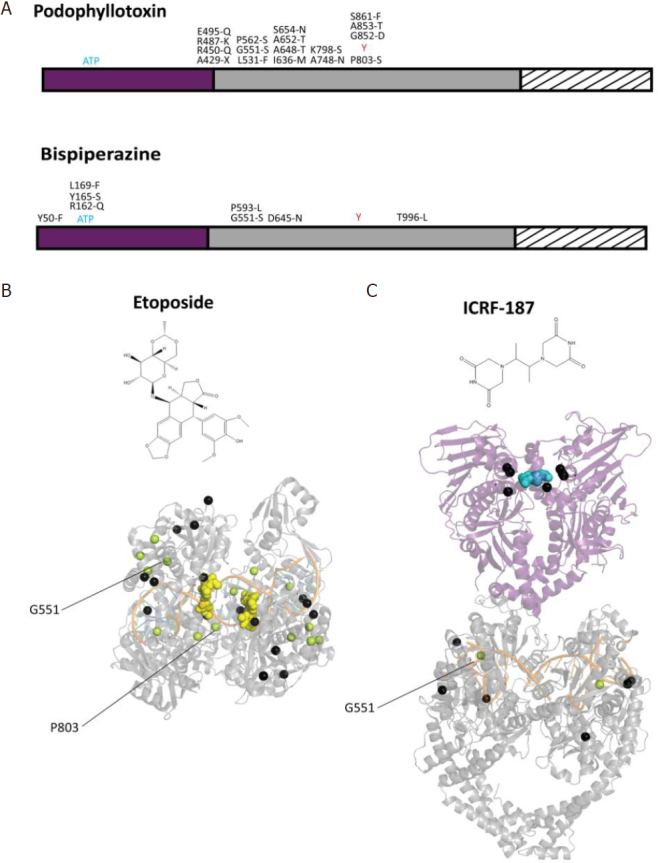
Distribution of point mutations providing drug resistance on the structures of the Top2α catalytic domains. A: distribution of the positions presenting single point mutations that confer resistance to podophyllotoxin compounds (etoposide) (top) and bispiperazine (ICRF-187) (bottom), indicated on the domain diagram of Top2α. B: single point mutations conferring resistance to anti-Top2 drugs are represented as black spheres reported on the structures of the catalytic domains. The DNA binding/cleavage domain (PDBID: 5GWK) of human Top2α homolog bound to two etoposide molecules represented as yellow spheres (B). The ATPase domain of the yeast homolog (PDBID: 1QZR) bound to the ICRF-187 compound represented as blue spheres, with mutations also appearing in the DNA binding/cleavage domain (PDBID: 5GWK) (C). Residues mutated to serine in resistant cell lines appear in pale green. Positions specifically mentioned in the main text are indicated by a black line
