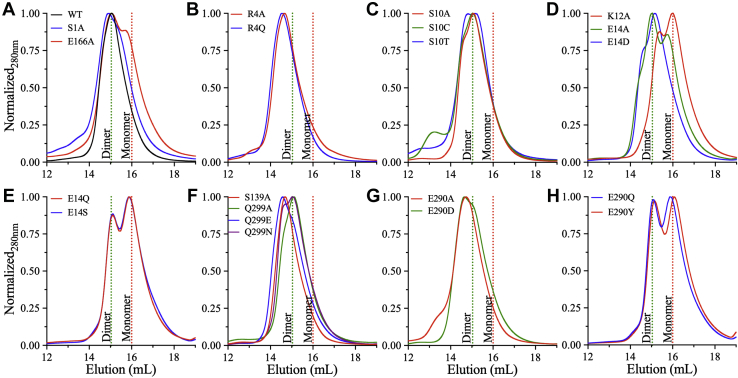
Figure 2.
Analytical size-exclusion chromatography (aSEC) of WT and dimer interface mutants of 3CLpro.A, gel filtration profiles (Superdex 200 Increase 10/300 GL; Cytiva Life Sciences/Biacore) of WT (black), S1A (blue), and E166A (red) indicating dimer formation. A shoulder corresponding to the monomer is evident for E166A. B, aSEC profiles of Arg4 substitutions. R4A (red) and R4Q (blue) were mostly dimer. C, aSEC profiles of S10A (red), S10C (green), and S10T (blue) indicating dimer formation with retention times similar to that of WT. D, gel filtration profiles of mutants in the one-turn α-helix of the N-finger. E14D (blue) was a dimer. K12A (red) and E14A (green) showed an equilibrium between two states with a preference for the monomer or dimer, respectively. E, aSEC profiles of E14Q (red) and E14S (blue) showing an equilibrium between monomeric and dimeric states with a preference for the monomer. F, aSEC profiles of S139A (red), Q299A (green), Q299E (blue), and Q299N (purple) indicating dimer formation. The retention volume of Q299A/N was similar to that of WT. By contrast, a major peak with a smaller retention volume than WT was observed for S139A or Q299E. G, gel filtration profiles of E290A (red) and E290D (green) indicating dimer formation with a smaller retention volume of the major peak compared with WT. H, aSEC profiles of E290Q (blue) and E290Y (purple) exhibited an equilibrium between the dimer and monomer. The vertical dashed lines on all panels are retention volumes of dimeric (green) and monomeric (red) forms of the WT enzyme, which have molecular weights of 34.5 and 69 kDa, respectively. The enzyme concentration for all runs was >7 mg/ml in 20 mM Hepes buffer, pH 7.0, with temperature of 4 °C. Data are representative of triplicate runs. 3CLpro, 3C-like protease.