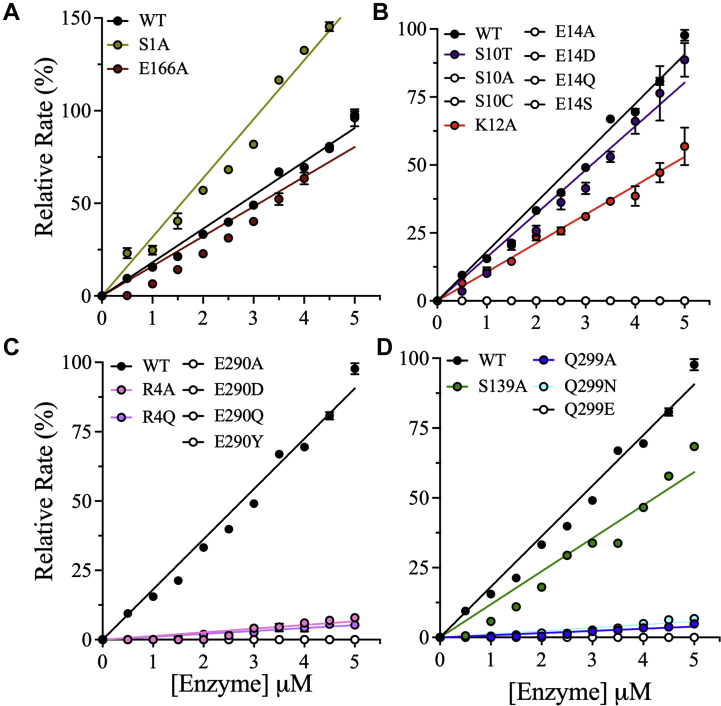
Figure 4.
Effects of dimer interface mutations on the relative activity of 3CLpro.A–D, the relative enzymatic activities of the dimer interface mutants of 3CLpro were measured at different enzyme concentrations up to 5.0 μM. The assays were performed in 20 mM Hepes (pH 7.0), 150 mM NaCl, 1 mM EDTA, 1 mM TCEP, and 20% (v/v) DMSO at a fixed peptide substrate concentration of 60 μM. The relative rate was calculated by normalization to the rate of WT, which was set to 100%. The data for mutants with no enzymatic activity are shown as open black circles. The data for enzymatically active mutants are color coded, and the data for WT are shown as filled black circles. Data points are means ± SD of triplicate measurements. 3CLpro, 3C-like protease; DMSO, dimethyl sulfoxide; TCEP, Tris(2-carboxyethyl)phosphine.