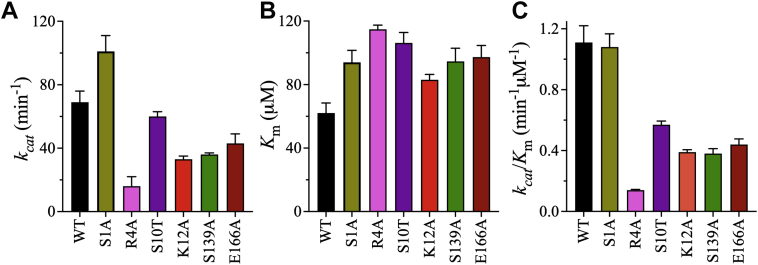
Figure 5.
Effects of dimer interface mutations on the kinetic parameters of 3CLpro.A and B, bar plots of kcat and Km values of WT and dimer interface mutants of 3CLpro. All mutants exhibited decreased kcat except S1A, which had higher kcat than WT. In addition, all mutants had reduced affinity for the peptide substrate, as indicated by increases in their Km values compared with WT. C, bar plot of the catalytic efficiency (kcat/Km) of the dimer interface mutants of 3CLpro. R4A had the lowest catalytic efficiency, whereas the catalytic efficiency of S1A was equivalent to that of WT. Thus, these residues have different impacts on the activity of 3CLpro despite their proximity in the N-finger. Data points are means ± SD of triplicate measurements. 3CLpro, 3C-like protease.