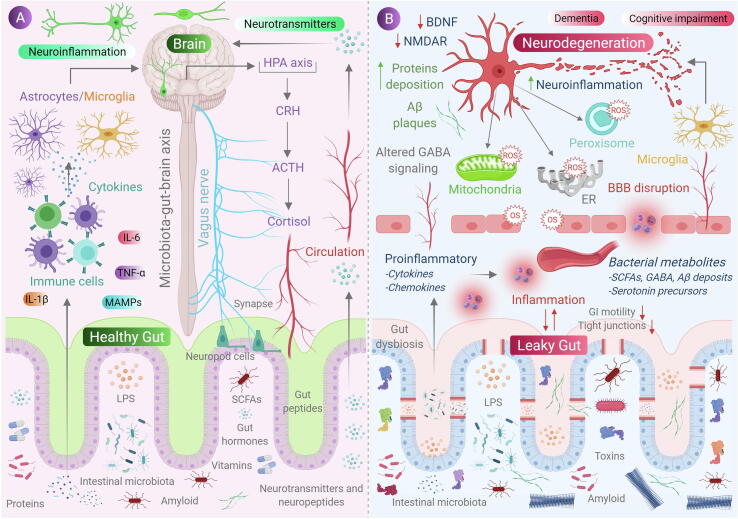
Fig. 1.
Role of gut microbiota in neurodegeneration. (A) Communication between gut and the brain involves neural, metabolic, endocrine and immunological pathways. Gut microbial molecules like neurotransmitters, amino acids, short-chain fatty acids (SCFAs), amyloid, lipopolysaccharide (LPS) and microbe-associated molecular patterns (MAMPs) interacts with host immune system via circulation affecting metabolism and the nervous system of the host and, also it affects the brain by direct activation of the vagus nerve via enteric nervous system to the brain. Conditions like stress causes the hypothalamic neurons to secrete corticotrophic receptor harmone (CRH), triggering the release of adrenocorticotrophic releasing hormone (ACTH), subsequently activating the release of cortisol, which affects intestinal barrier integrity affecting gut health. (B) when, there occurs a condition of gut dysbiosis, there is a decrease of anti-inflammtory molecules (like SCFAs, H2) to that of pro-inflammatory molecules (LPS, amyloids) also with an alteration of beneficial bacteria to that of pathogenic in the gut. This results in increase of intestinal and blood brain barrier permeability, subsequently causing increase in peripheral immune responses, thus increasing oxidative stress in central nervous system (CNS). An increased production of reactive oxygen species (ROS) can be observed in cell organelles like mitochondria, endoplasmic reticulum (ER) and peroxisomes in neurons, along with neurotoxin aggregation, resulting in neurodegeneration.