Fig. 3. RBD-dependent C3 deposition on nanoparticles.
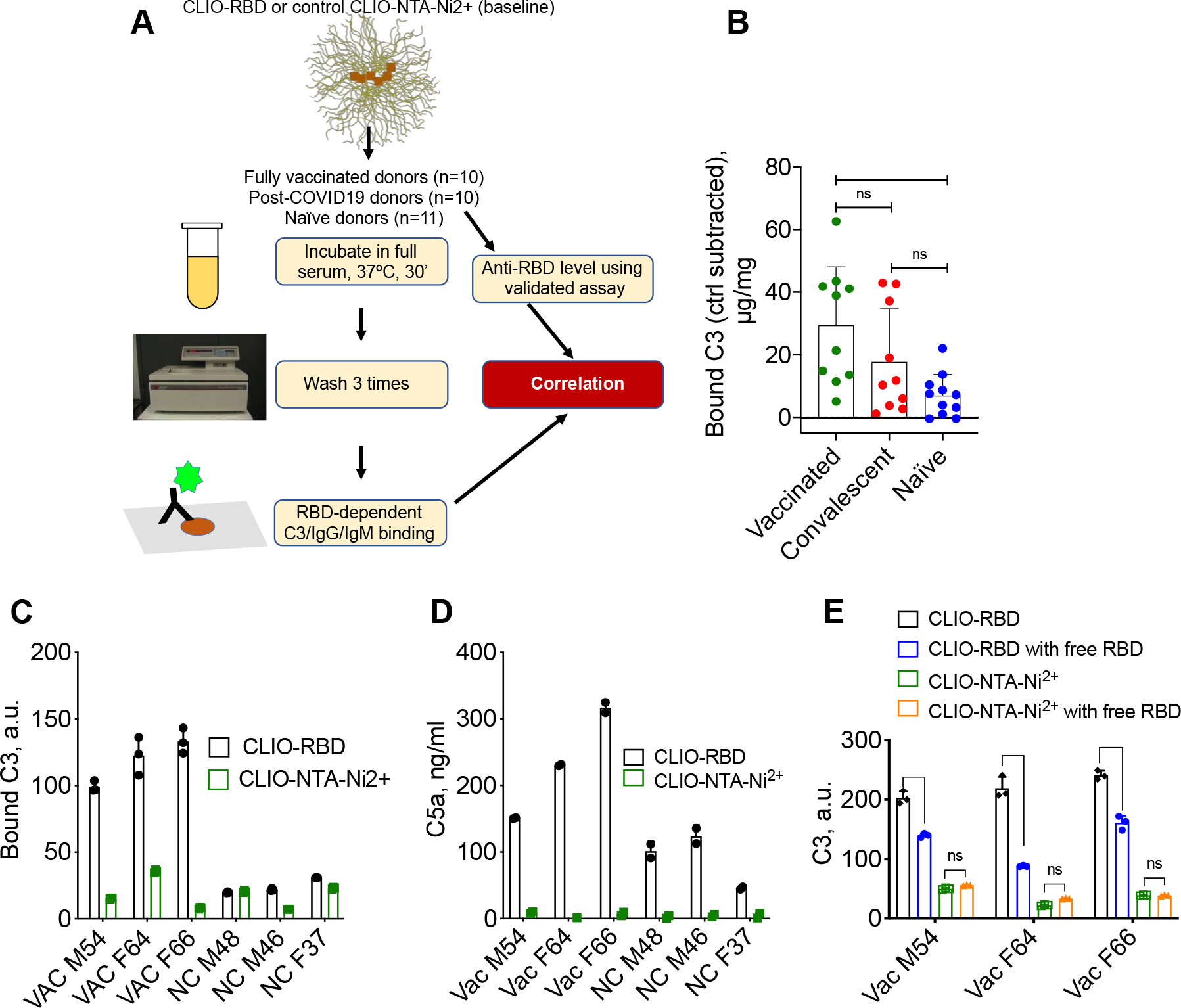
A) Study design. C3, IgG, and IgM binding were quantified by dot-blot assay; B) Levels of bound C3 (μg C3/mg Fe, each dot is the mean value of 3 technical replicates) were calculated after subtracting C3 deposition on control CLIO-NTA-Ni2+ particles. Full raw data are in Supplementary Fig. S1. On average, the deposition was increased in vaccinated sera compared to naïve sera (p-value = 0.0047, statistical analysis in Supplementary Table 2). Only some of the vaccinated and convalescent samples had higher RBD-dependent C3 deposition; C-D) Deposition of C3 (C) and release of fluid phase marker C5a (D) after incubation of CLIO-RBD and CLIO-NTA-Ni2+ in vaccinated (VAC) and naïve (NC) sera (means of 3 technical replicates). Both assays demonstrate RBD-dependent complement activation, enhanced in vaccinated sera; E) C3 deposition on CLIO-RBD is decreased in the presence of 0.2 mg/mL soluble RBD protein (means of 3 technical replicates, ****p<0.0001).
